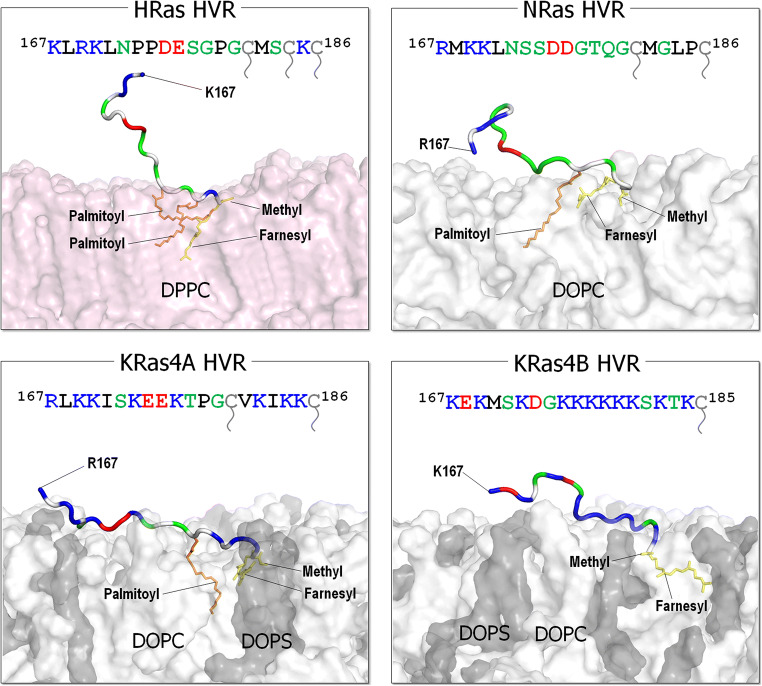
Fig. 1.
The sequences of C-terminal hypervariable regions (HVRs, residues 167–185/186) of the Ras isoforms and the modeled HVR structures with the post-translational modifications (PTMs) representing their preferred membrane interactions. The modeled HRas and NRas HVR peptides preferentially bind the zwitterionic DPPC or DOPC bilayers in the gel or liquid phases, while the KRas HVR peptides anchor to the anionic DOPC/DOPS bilayer in the liquid phase. In the HVR sequence, basic residues (positively charged) are colored in blue, acidic residues (negatively charged) are colored in red, hydrophobic residues are colored in black, and polar and glycine residues are colored in green. The prenylated Cys residues are colored in gray with a tail mark. In the HVR cartoon, the same colors are used, except the hydrophobic residues colored in white. The farnesyl and palmitoyl groups are shown as yellow and orange sticks, respectively. In the surface representation of lipid bilayer, pink, white, and gray surfaces denote DPPC, DOPC, and DOPS, respectively