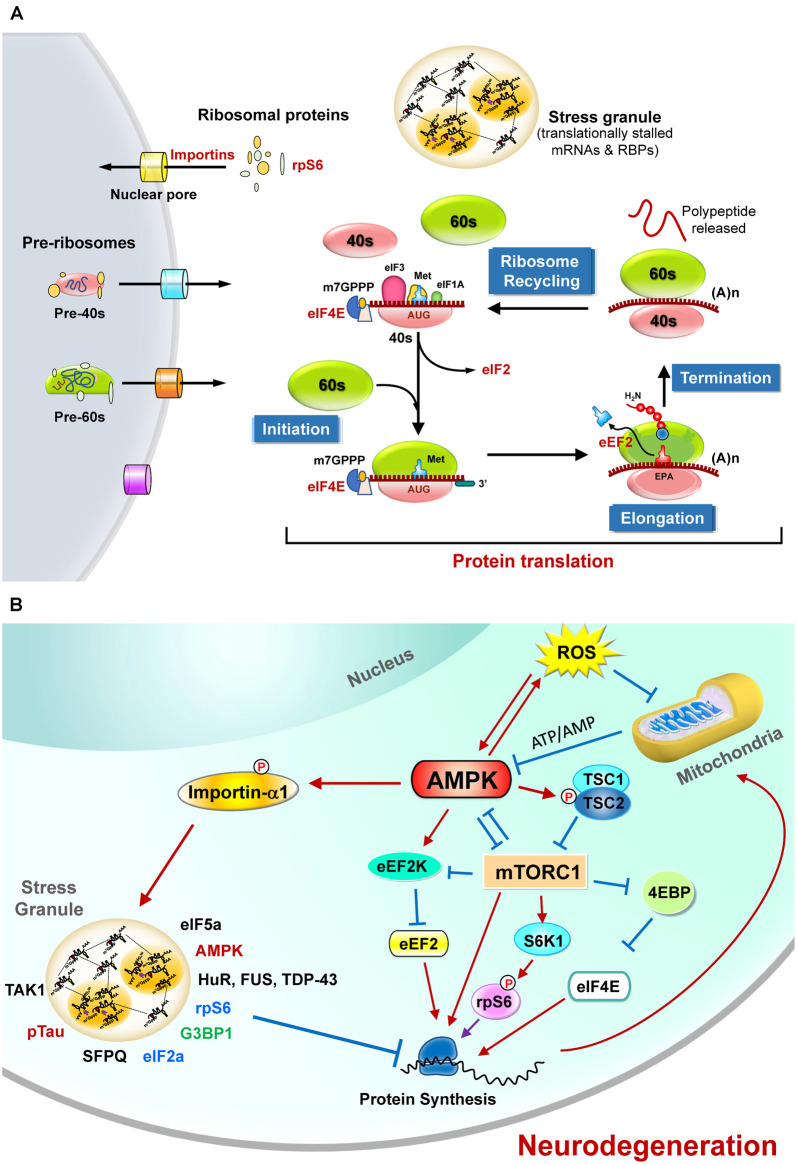
Figure 1.
The regulation of protein translation by energy deficiency and AMPK activation at multiple steps. (A) Schematic representation of the four critical steps to control eukaryotic protein translation. For the sake of clarity, only those sensitive to energy homeostasis are listed here. The initiation stage involves the start codon recognition and the joining of the ribosomal subunits (40S and 60S), followed by the elongation stage with repetitive binding of aa-tRNA and translocation. When the ribosome complex encounters stop codons (UAA, UGA, or UAG), protein synthesis is terminated with the help of a protein release factor at the termination stage. The ribosome complex is then dissociated into ribosomal subunits in the ribosome recycling stage and transported into nuclei by importins. These steps are controlled by hundreds of proteins including various initiation factors (eIF) and elongation factors (eEF). In response to stress stimuli that reduce protein translation efficiency, the formation of stress granules (SGs) containing translationally stalled mRNAs and RNA-binding proteins (RBPs) can be observed. (B) The protein translation process is regulated by mitochondrial defect and AMPK activation. Briefly, elevated reactive oxygen species (ROS) and the mitochondrial defect may cause AMPK activation and subsequently inhibit protein translation by suppressing the functions of eIF4E, rpS6, s and eEF2. Activation of AMPK also causes phosphorylation of importin-α1 and interrupts its function in mediating nucleocytoplasmic transport of several RBPs (including TDP-43, HuR). During these stresses, importin-α1 can be found in SGs and is believed to play an important role in the assembly of SGs for the temporary repression of protein translation. A wide variety of proteins (including the components of translation apparatus, RBPs, ribosomal proteins, and pTau) are also recruited to SGs under stresses that cause neurodegeneration. See text for additional details.