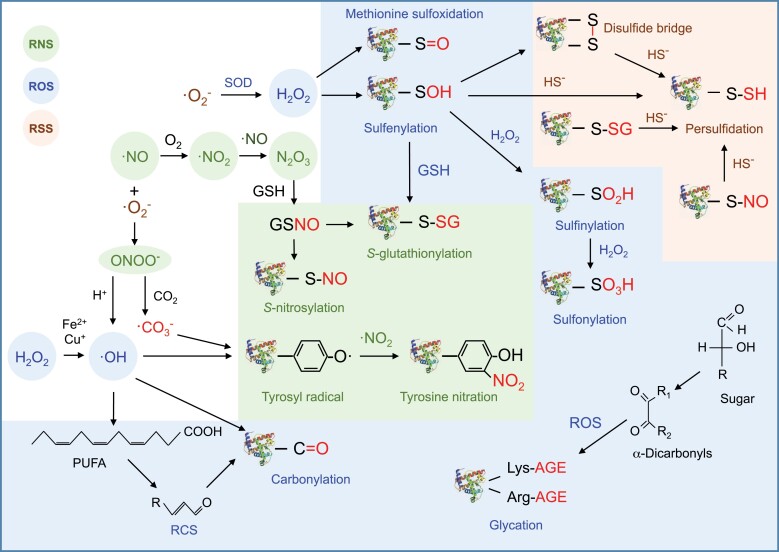
Fig. 2.
Redox-dependent PTMs. Met residues can be oxidized by hydrogen peroxide (H2O2) to Met sulfoxides. The S and R stereoisomers are specifically reduced back to Met by methionine sulfoxide reductases A and B, respectively. Oxidation of deprotonated thiols of Cys residues (–S-) by H2O2 leads to the formation of sulfenic acid (–SOH), which may react with another thiol to form disulfides (–S–S–). This modification can be reverted by thioredoxins and glutaredoxins. The –SOH group can be an intermediate to other redox modifications (see below) or be further oxidized to sulfinic acid (–SO2H) and sulfonic acid (–SO3H). S-nitrosylation (–SNO) is mostly mediated by nitrogen oxides and trans-nitrosylating agents such as S-nitrosoglutathione (GSNO). S-glutathionylation (–SSG) occurs by two main mechanisms: reaction of the target protein with GSNO, and reaction of reduced glutathione (GSH) with –SOH. The reaction of hydrogen sulfide (HS-) with –SOH, –SNO, –SSG, or disulfide bridges induces persulfidation (–SSH). Peroxynitrite (ONOO-) is formed by the reaction of nitric oxide (NO) with superoxide (O2.-) radicals. In turn, radicals derived from ONOO- breakdown oxidize Tyr residues to tyrosyl radicals; these react with nitrogen dioxide (NO2), produced from ONOO- decomposition, to yield NO2–Tyr. The direct oxidation of Lys, Arg, Pro, and Thr by hydroxyl radicals (·OH) incorporates the carbonyl moiety into proteins. Alternatively, oxidation of a polyunsaturated fatty acid (PUFA; a simplified representation is shown lacking part of the aliphatic chain) produces unstable lipid hydroperoxides that decompose to secondary products known as reactive carbonyl species (RCSs). These react with amino acid side chains and generate carbonyl derivatives. Moreover, Arg and Lys residues may react with reducing sugars or α-dicarbonyls such as glyoxal and methylglyoxal, generating glycation products that are readily oxidized to form relatively stable advanced glycation end products (AGEs).