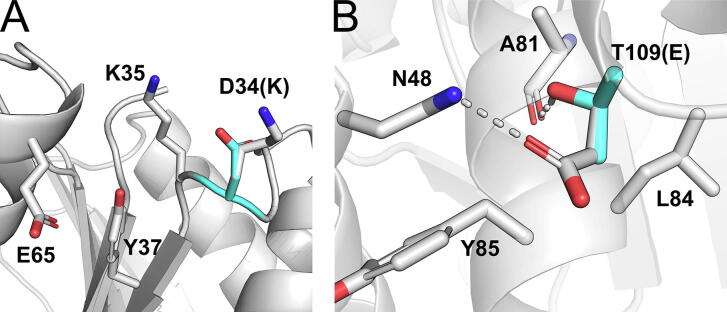
Fig. 6.
Potential resistance mechanisms of the aIL-resistant D34K and T109E variants. (A) Residues involved in intramolecular interactions with D34 (cyan sticks) or K34 (white sticks) are shown as sticks. In the D34K variant, the introduced lysine residue most likely repels [BMIM+] ions around the residue patch, preventing potential conformational changes introduced upon binding of [BMIM+] to D34 and its adjacent residues. (B) Residues involved in intramolecular interactions with T109 (cyan sticks) or E109 (white sticks) are shown as sticks. In the T109E variant, the hydrogen bond between T109Oγ and the backbone oxygen of A81 with a distance of 1.8 Å is replaced with a strong ionic interaction of E109Oε with N48Nε (distance of E109Oε to N48Nε is 2.8 Å). The introduced negative surface charge most likely also reduces the occurrence frequency of [TfO–] ions around the residue patch. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)