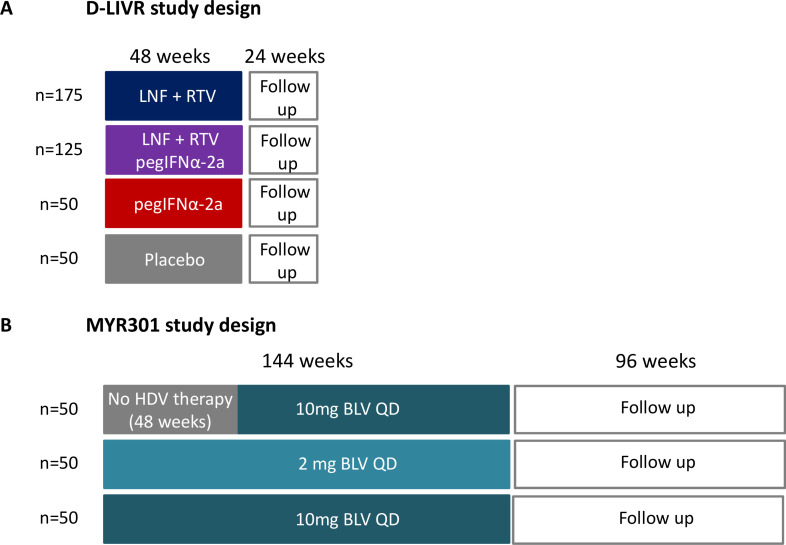
Figure 5.
Study design of the two ongoing phase III studies assessing the efficacy and safety of new therapeutic regimens against HDV. (A) D-LIVR study. LNF +RTV: LNF 50 mg two times per day+RTV 100 mg two times per day. Primary endpoint: ≥2 log10 IU/mL decline in HDV RNA and ALT normalisation in week 48. All patients will be maintained on background HBV nucleoside/nucleotide analogue therapy. (B) MYR301 study. primary endpoint: undetectable HDV RNA or decrease by ≥2log10 IU/mL and ALT normalisation in week 48. If indicated treatment with nucleoside/nucleotide analogues according to European Association for the Study of the Liver (EASL)/American Association for the Study of the Liver (AASLD) guidelines. ALT, alanine aminotransferase; BLV, bulevirtide; HBV, hepatitis B virus; HDV, hepatitis D virus; LNF, lonafarnib; pegIFNα, pegylated interferon-α; RTV, ritonavir;