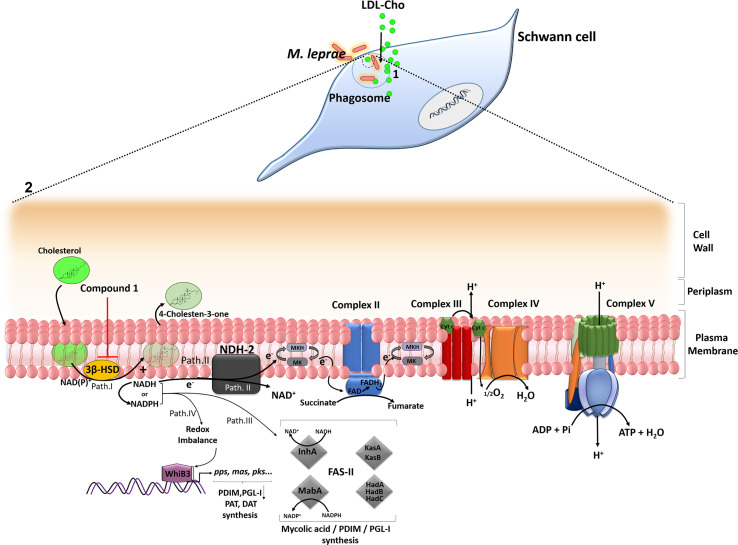
Figure 6.
A model proposing a key role of cholesterol oxidation for M. leprae survival inside SCs. (1) After internalization, M. leprae induces LDL-cholesterol (LDL-Cho) uptake that is recruited to bacterium-containing phagosomes. (2) Magnification of M. leprae cell envelope. Path I - 3β -HSD oxidizes cholesterol to cholestenone generating NADH, which can be converted to NADPH. Path II - NADH derived from 3β-HSD feeds bacterial electron respiratory chain via type-II NADH dehydrogenase (NDH-2), which reduces menaquinone (MK) to menaquinol (MKH), a substrate of the succinate dehydrogenase (complex II). The proton motive force generated during electron transport chain will be used by the ATP synthase (complex V) to generate ATP. Path III – NADH/NADPH can be used by fatty acid synthase II (FASII) for the synthesis of PDIM and PGL-I. The FASII complex trans-2-enoyl-AcpM reductase (InhA) and β-ketoacyl-AcpM reductase (MabA) subunits use NADH and NADPH, respectively. Path IV - WhiB3, a probable transcriptional regulatory protein, senses variations in the redox balance through NADH and NADPH levels and activates the promoter region of polyketide biosynthetic genes inducing the synthesis of mycobacterial lipids such as polyacyltrehaloses (PAT), diacyltrehaloses (DAT), PDIM and PGL-I. Inhibition of 3β -HSD by compound 1 reduces the levels of NADH/NADPH affecting the metabolic pathways described and impacts M. leprae intracellular viability. The arrows indicate some of the destinations of NAD(P)H already described in the literature and propose routes fed by the reducing power generated from 3β–HSD.