Figure 6.
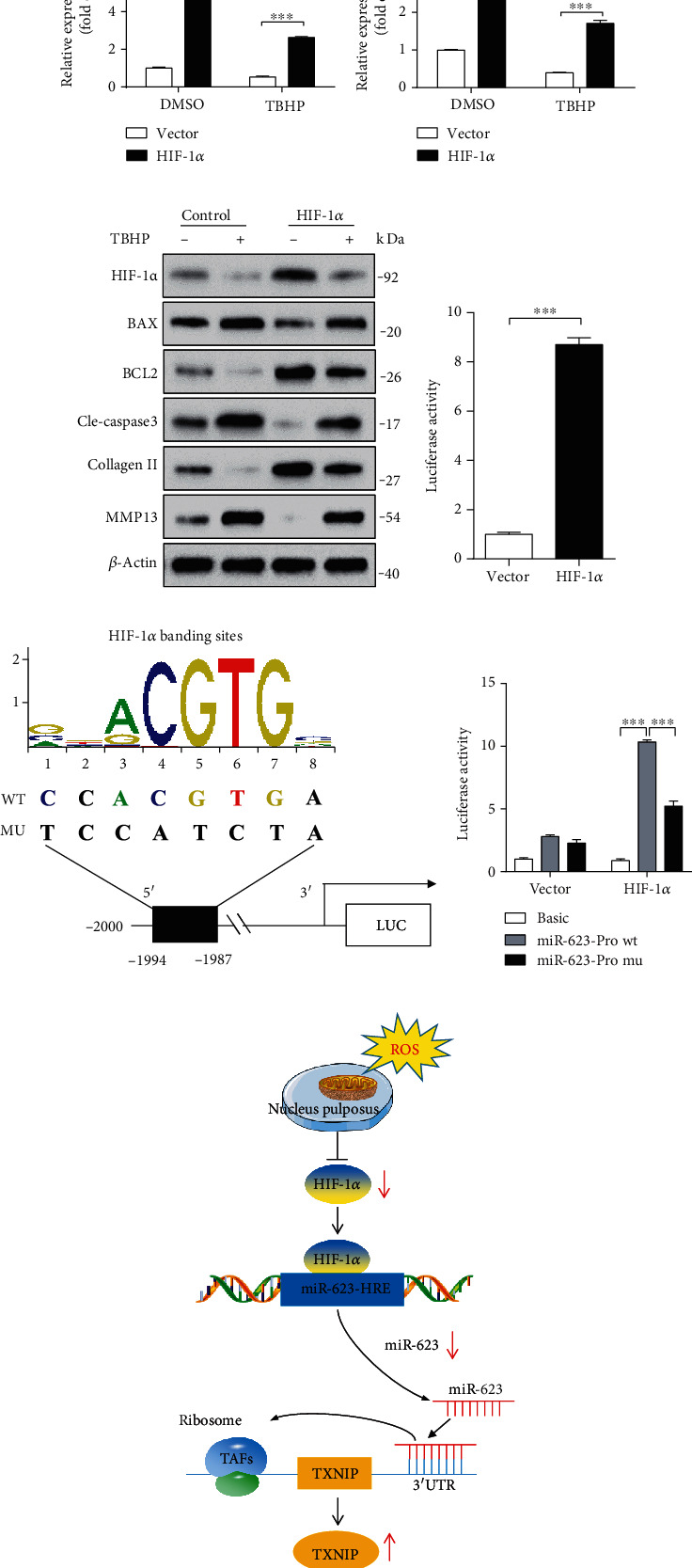
miR-623 expression is regulated by HIF-1α under oxidative stress. (a) Western blot analysis of HIF-1α expression in normal and degenerated NP tissues. (b, c) Quantification analysis of HIF-1α and miR-623 expression in NP cells induced by TBHP with or without HIF-1α overexpression. (d) Western blot analyses of HIF-1α, BAX, BCL2, cleaved caspase-3, collagen II, and MMP13 in NP cells induced by TBHP with or without HIF-1α overexpression. (e) miR-623 promoter activity induced by HIF-1α overexpression. (f) The schematic of the WT and mutant miR-623 promoter constructs. (g) miR-623 promoter activity induced by HIF-1α with or without putative HIF-1α binding site mutation on the miR-623 promoter. (h) The schematic graph reflects the HIF-1α-miR-623-TXNIP pathway in NP cell apoptosis and inflammatory responses induced by oxidative stress. Normally, NP is an avascular tissue under a hypoxic environment and expresses a high level of HIF-1α. Under oxidative stress conditions, HIF-1α expression is dampened and results in decreased nuclear translocation to bind to the hypoxia-reactive element on the miR-623 promoter, leading to decreased miR-623 transcription. The dampened miR-623 expression alleviates the inhibited effect miR-623 on TXNIP expression by targeting to its 3′-UTR, ultimately leading to increased apoptosis and inflammatory responses of NP cells and initiating the IVDD. ∗∗∗P < 0.001. P values were analyzed by two-tailed t-tests in (e) and two-way ANOVA in (b, g).
