Abstract
Posttranslational modification of protein by lysine-48 (K48) linked ubiquitin (Ub) chains is the major cellular mechanism for selective protein degradation that critically impacts biological processes such as cell cycle checkpoints. In this chapter, we describe an in vitro biochemical approach to detect a K48-linked di-Ub chain by fluorescence resonance energy transfer (FRET). To this end, we detail methods for the preparation of the relevant enzymes and substrates, as well as for the execution of the reaction with high efficiency. Tracking K48 polyubiquitination using this sensitive and highly reproducible format provides an opportunity for high-throughput screening that leads to identification of small molecule modulators capable of changing ubiquitination for improving human health.
Keywords: K48 ubiquitination, Cullin-RING E3 ubiquitin ligase, E2 Cdc34, Fluorescently labeled ubiquitin, FRET
1. Introduction
Timely removal of cell cycle regulators by ubiquitin (Ub)-dependent proteolysis is fundamental to the functioning of cell division cycle [1]. Central to selective protein turnover by the 26S proteasome is the formation of homotypic Lysine-48 (K48) linked Ub chains that tag substrate proteins for degradation [2]. The underlying biochemical process, termed ubiquitination, is driven by three enzymes: an E1 activating enzyme, an E2 conjugating enzyme, and an E3 ligase that recognizes a substrate and hence governs the specificity of the reaction [2]. One well-characterized K48-polyubiquitination system involves E3 Cullin-RING Ub ligase 1 (CRL1; also named as SCF [Skp1-Cullin1-F-box-ROC1/Rbx1] complex) and E2 Cdc34 [3, 4]. CRL1 substrates include many key cell cycle modulators, such as p27 and cyclin E [5].
To track CRL1-mediated K48 ubiquitination by fluorescence, we developed a “fluorescence (Förster) resonance energy transfer (FRET) K48 di-Ub assay” [6]. The assay is composed of five components: (1) E1, (2) E2 Cdc34 specific for K48 linkage, (3) E3 sub-complex ROC1-Cullin 1 (CUL1) (411–776) (called ROC1-CUL1 CTD) required for E2 activation, (4) donor Ub (carrying K48R substitution and fluorescent dye iFluor 555 at position Q31C; Fig. 1), and (5) receptor Ub (bearing G75G76 deletion and fluorescent dye iFluor 647 at position E64C; Fig. 1). The reaction has been modified from previously reported versions [7], allowing for only one nucleophilic attack to produce a single Ub–Ub isopeptide bond (Fig. 2) exclusively carrying a K48-linkage [8]. In this ubiquitination system, ROC1 binds to the Cdc34 catalytic core domain [9], whereas the CUL1 CTD provides a basic pocket that interacts with Cdc34’s acidic C-terminus, thereby facilitating E2 recruitment [10].
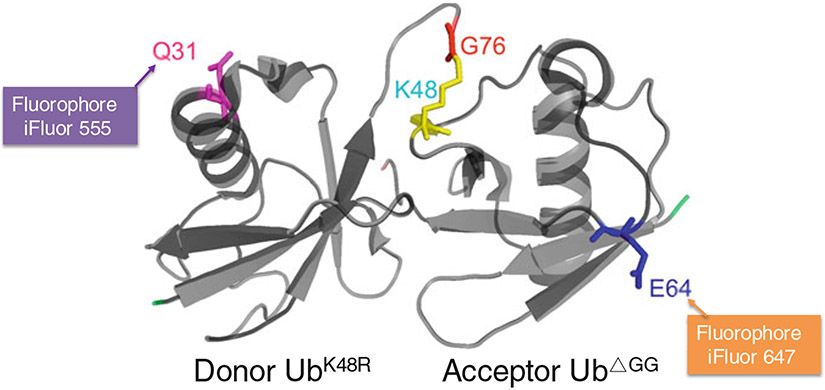
Fig. 1.
Conjugation of fluorescent dye to a pair of residues on the donor and acceptor Ub. A K48 di-Ub chain is shown based on previous crystallographic studies [16] (PDB 1F9J). At position Q31 or E64 of the donor or acceptor Ub, respectively, each residue is substituted by cysteine and labeled with the indicated fluorescent dye. For iFluor 555, Exmax is 559 nm and Emmax is 569 nm. For iFluor 647, Exmax is 654 nm and Emmax is 674 nm. The calculated distance between Ub C31-linked iFluor 555 and Ub C64-linked iFluor 647 in the K48 di-Ub chain is 38 Å. This distance is within the range of the Förster radius (Ro) for iFluor 555/iFluor 647, which is about 51 Å (at this distance the resonance transfer efficiency is 50%)
Fig. 2.
FRET K48 di-Ub assay Scheme. E1 and UbQ31C/K48R-iFluo 555 form a thiolester complex. Transthiolation then follows to yield Cdc34~UbQ31C/K48R-iFluo 555. In the presence of E3 sub-complex ROC1-CUL1 CTD, the K48 residue of UbE64C/ΔGG-iFluo 647 attacks Cdc34~UbQ31C/K48R-iFluo 555. Consequently, the resulting di-Ub brings iFluo 555 and iFluo 647 into proximity that generates FRET upon donor fluorophore excitation
The “FRET K48 di-Ub assay” was effectively used for a high-throughput screen (HTS) campaign to discover small molecule chemical probes that target E3 CRL1 [6]. The restriction to a single Ub–Ub linkage eliminates complexity associated with polyubiquitin chains, ensuring a high degree of reproducibility that is critical to HTS success. Secondly, FRET is produced by two Ub-linked fluorophores that become juxtaposed upon Ub–Ub conjugation (Fig. 2). Each fluorophore is uniquely engineered to either donor or receptor Ub at specific site (Fig. 1). Note that optimal positioning of fluorophore in Ub is critical, as N-terminally labeled Ub, commonly used in previous publications [11, 12] is inactive in our system. Commercial fluorescent Ub proteins do not specify the location of the fluorescent dye in Ub and it is unclear if such reagents can be generally optimal for HTS FRET assays. On the other hand, this FRET strategy should be applicable to other HTS campaigns that depend on K48 ubiquitination, but require different E3/E2 enzyme combinations.
The method detailed here provides a FRET-based strategy for accurately quantifying K48 polyubiquitination, and can be used as a platform to discover and characterize small molecule agents capable of perturbing ubiquitination.
2. Materials
2.1. Preparation of Ub, E1, and E2 Cdc34 (See Note 1)
pHisTEVyUb-Q31C/K48R (see Note 2), pHisTEVyUb-E64C/ΔGG (see Note 3), pET3a-Uba1 (E1)-His10 (see Note 4), or pET28a-His6-TEV Cdc34 C191S/C223S (see Note 5) expression plasmid.
Escherichia coli Rosetta 2(DE3)pLysS cells, stored at −80 °C (EMD Millipore).
Luria–Bertani (LB) Broth Base, diluted to 15.5 g/L, auto-claved, and stored at 4 °C (Sigma-Aldrich).
Carbenicillin, stock is 100 mg/mL in H2O, and stored at −20 °C (Sigma-Aldrich).
LB Agar Plates + carbenicillin (100 μg/mL) (Sigma-Aldrich). Isopropyl β-d-1-thiogalactopyranoside (IPTG), stock is 1 M in H2O (Sigma-Aldrich).
Lysis buffer: 20 mM Tris–HCl, pH 8.0, 1% Triton X-100,0.5 M NaCl, 2 mM phenylmethylsulfonyl fluoride, 0.4 μg/mL antipain, and 0.2 μg/mL leupeptin).
Buffer A: 25 mM Tris–HCl (pH 7.5), 0.01% Nonidet P-40 (NP-40), 10% glycerol, 1 mM dithiothreitol (DTT), 0.1 mM PMSF, and 0.2 μg/mL of antipain and leupeptin).
Imidazole wash buffer: Buffer A (2.1.7) + 50 mM NaCl + 5 mM imidazole.
Imidazole elution buffer: Buffer A (2.1.7) + 50 mM NaCl + 250 mM imidazole.
Ni-nitrilotriacetic acid-agarose (Ni-NTA) beads (Qiagen). Dialysis buffer: 25 mM Tris–HCl, pH 7.4, 10% glycerol, 50 mM NaCl, 0.01% NP-40, and 1 mM DTT.
SnakeSkin Pleated Dialysis Tubing, 3500 MWCO (Pierce).
Mono Q HR 10/10 column (Amersham Biosciences).
Amicon Ultra-15 Centrifugation Filters, 10 K (Millipore).
Sephadex-75 gel filtration column (Amersham Biosciences).
AKTA FPLC (GE Healthcare).
2.2. Preparation of E3 ROC1-CUL1 CTD Complex
pETDuet-1-MCS-I-CUL1 (L421E, V451E, V452K, Y455K)/MCS-II-ROC1 expression plasmid (see Note 6).
BL21-CodonPlus(DE3)-RIL cells, stored at −80 °C (Agilent). Wash buffer (50 mM Na2HPO4, 300 mM NaCl, 10 mM imidazole, pH 8.0).
EDTA-free protease inhibitor tablet (Roche).
EmulsiFlex-C5 homogenizer (Avestin, Ottawa, Ontario).
Filter (0.45μm, Millipore, Mississauga, ON, Canada).
HisTrap FF column (GE Healthcare).
Elution buffer (50 mM Na2HPO4, 300 mM NaCl, and 250 mM imidazole, pH 8.0).
2.3. Preparation of Fluorescently Labeled Ub
Anaerobic glove box (Captair Pyramid, Erlab) under nitrogen gas.
TCEP (Sigma).
Phosphate-buffered saline (PBS).
iFluor 555 maleimide and iFluor 647 maleimide (AAT Bioquest).
DMF (Sigma).
2.4. FRET K48 di-Ub Assay
Costar 384 well storage plate #3658 (Corning).
Ub E1 (Subheading 3.1), stored at −80 °C.
E2 Cdc34 (Subheading 3.1), stored at −80 °C.
ROC1-CUL1 CTD (Subheading 3.2), stored at −80 °C.
Ub C31-iFluor 555 (Subheading 3.3) and Ub C64-iFluor 647 (Subheading 3.3), stored at 4 °C.
Centrifuge with adaptor for microtiter plate (Eppendorf).
Synergy-H1 reader (BioTek).
2.5. Running the Gel
NOVEX 4–20% Bis-Tris Protein Gel (12–15 well, 1.0 mm).
10× Tris/Glycine/SDS Running Buffer, diluted to 1× with distilled H2O (Bio-Rad).
Protein marker (Bio-Rad).
Power supply (Bio-Rad).
4× SDS loading buffer: 40% glycerol, 240 mM Tris–HCl (pH 6.8), 8% SDS, and 0.04% bromophenol blue dissolved in H2O. Coomassie Brilliant Blue solution: Coomassie R-250 (Bio-Rad), methanol (30% v/v), glacial acetic acid (10% v/v).
Destaining solution: methanol (10% v/v), glacial acetic acid (10% v/v).
2.6. Fluorescence Imaging
Typhoon FLA 9500 laser scanner (GE).
3. Methods
3.1. Preparation of Ub, E1, or E2 Cdc34
Transform pHisTEVyUb-Q31C/K48R, pHisTEVyUb-E64C/ΔGG, pET3a-Uba1 (E1)-His10, or pET28a-His6-TEV Cdc34 C191S/C223S plasmid into Escherichia coli Rosetta 2(DE3)pLysS cells. Incubate cells on ice for 30 min, heat-shock at 37 °C for 30 s, and return to ice for 2 min. Plate on LB agar plates with carbenicillin.
Pick a single colony and inoculate in a starter culture overnight in LB + 100 μg/mL carbenicillin (see Note 7).
Add the starter culture to the growth culture (LB with 0.4% glucose in the presence of carbenicillin) in a 1:100 ratio of dilution. Grow at 37 °C until the optical density measured at 600 reaches 0.5 (~3–3.5 h).
Induce culture with IPTG at a final concentration of 1 mM overnight at 25 °C.
Pellet bacterial cells by centrifugation at 5000 x g for 15 min at 4 °C.
Resuspend cells in 1/25 the culture volume of lysis buffer (Subheading 2.1, item 6).
Sonicate the lysate (4× 20 s) and centrifuge it at 10,000 × g for 30 min.
Mix soluble extracts with Ni-nitrilotriacetic acid-agarose beads (Ni-NTA) beads, at a ratio of 0.3 mL beads per 1 mL extracts, and rotate the mixture for 2 h at 4 °C.
Pack beads into a Flex-Column and wash with buffer A (Subheading 2.1, item 7) + 50 mM NaCl, in 10× the column volume, and imidazole wash buffer (Subheading 2.1, item 8), in 3× the column volume.
Elute protein with imidazole elution buffer (Subheading 2.1, item 9), in 10× the column volume, collecting 1 mL fractions.
Determine peak fractions by subjecting aliquots of the fractions (1μL) to SDS-PAGE (Subheading 2.5). Stain the gel for 10 min in Coomassie solution (Subheading 2.5, item 5), rinse with distilled water, and destain in destaining solution (Subheading 2.5, item 6).
Pool peak fractions, load into dialysis tubing prerinsed with distilled water, and properly seal the ends. Dialyze twice, 5 h to overnight each time, by placing the tubing in 10–20× the volume of dialysis buffer at 4 °C (Subheading 2.1, item 10).
No further steps are necessary for Ub-Q31C/K48R or Ub-E64C/ΔGG. The yield is approximately 83 or 30 mg of purified Ub-Q31C/K48R or Ub-E64C/ΔGG (from 1 L culture), respectively. They are stored at −80 °C.
To further purify E1, load Ni-NTA-purified, dialyzed material onto a Mono Q HR 10/10 column using the AKTA FPLC. Wash column with buffer A + 50 mM NaCl.
Elute the protein with a 20 column volume linear gradient of 50–500 mM NaCl in buffer A (Subheading 2.1, item 7). Analyze by SDS-PAGE as in step 11 and pool the peak fractions.
Concentrate the peak fractions using centrifugal filters. The yield is approximately 4 mg of purified E1 from 1 L culture (see Note 8).
To purify Cdc34, follow steps 14–16. The expected yield for Cdc34 is approximately 18 mg from 1 L of culture (see Note 8).
3.2. Preparation of E3 ROC1-CUL1 CTD Complex
Transform pETDuet-1-MCS-I-CUL1 (L421E, V451E, V452K, Y455K)/MCS-II-ROC1 plasmid into BL21-Codon-Plus(DE3)-RIL cells. Incubate cells on ice for 30 min, heat-shock at 37 °C for 30 s, and return to ice for 2 min. Plate on LB agar plates with carbenicillin.
Pick a single colony and inoculate in a starter culture overnight in LB + 100 μg/mL carbenicillin + 34 mg/L chloramphenicol (see Note 7).
Add 10 mL of the starter bacterial colony to 4 L (1:400 dilution) of prewarmed LB media supplemented with 0.5 mM ZnCl2, 100 mg/L ampicillin, and 34 mg/L chloramphenicol. Grow at 37 °C at 210 rpm. When the culture reaches an OD600 of 0.4, the temperature is reduced to 16 °C with continued shaking.
Once the OD600 reached 0.7, the culture is induced with 1 mM IPTG and the cells are grown overnight.
Pellet the cells by centrifugation at 6000 × g for 10 min at 4 °C. Resuspend cells in 25 mL wash buffer with an EDTA-free protease inhibitor tablet.
Lyse cells using an EmulsiFlex-C5 homogenizer. If the mixture is still too viscous, pass the lysate through a syringe with a 21G syringe several times.
Clear lysates by centrifugation (110,000 × g for 1 h at 4 °C).
Filter the supernatant.
Load the filtered extracts onto a 5 mL HisTrap FF column preequilibrated with wash buffer at a flow rate of 0.5 mL/min using an AKTA FPLC.
Wash the column at 3 mL/min (15 column volumes with wash buffer containing 30 mM imidazole, then 10 column volumes with 60 mM imidazole).
Elute the ROC1-CUL1 CTD complex with elution buffer (Subheading 2.2, item 7) at a flow rate of 2 mL/min. Fractions containing the ROC1-CUL1 CTD complex, as determined by staining analysis following protein separation by SDS-PAGE, as in Subheading 3.1, step 11, were pooled.
Dialyze the pooled fraction as in Subheading 3.1, step 12.
To further purify ROC1-CUL1 CTD, proceed to steps 14–16 in Subheading 3.1.
The yield for the ROC1-CUL1 CTD complex is approximately 48 mg from 1 L of culture.
Protein aliquots are stored at −80 °C (see Note 8).
3.3. Preparation of Fluorescently Labeled Ub
Steps 1–4 manipulations are carried out inside an anaerobic glove box. To prepare fluorescent Ub, purified Ub-Q31C/K48R and Ub-E64C/ΔGG, 150 mg each, were treated with 10 mM TCEP in degassed phosphate-buffered saline (PBS) for 15 min at room temperature.
Add Ni-NTA agarose for 1 h at 4 °C in sealed tubes.
Wash the beads with degassed PBS to remove the TCEP and any unbound protein.
Incubate the resulting beads with 15 mg of either iFluor 555 maleimide for UbQ31C/K48R, or iFluor 647 maleimide for UbE64C/ΔGG), dissolved in DMF, for 1 h at room temperature then overnight at 4 °C, in the dark.
The subsequent steps were performed in normal air. Following incubation, beads were first washed with PBS to remove unreacted dye.
Elute the labeled proteins with PBS plus 250 mM Imidazole.
Dialyze the eluted proteins at 4 °C in the dark, against buffer A (Subheading 2.1, item 7) plus 150 mM NaCl. The final yield is ~108 mg or ~129 mg for labeled Ub-Q31C/K48R or Ub-E64C/ΔGG, respectively (see Note 9).
3.4. FRET K48 di-Ub Assay
Thaw all reagents on a metal block on ice (see Note 10).
The reaction mixture (15μL) is assembled onto a 384-well microtiter plate (or in a test tube). Each well contained 33 mM Tris–HCl (pH 7.4), 1.7 mM MgCl2, 0.33 mM DTT, BSA (0.07 mg/mL), Ub E1 (14 nM), E2 Cdc34 (124 nM), ROC1-CUL1 CTD (1μM), Ub C31-iFluor 555 (donor, 0.93μM), and Ub C64-iFluor 647 (receptor, 1.62μM) (see Note 11).
ATP (0.66 mM) is then added to the mix followed by a brief centrifugation to bring down the mixture.
The resulting plate is incubated at 30 °C in the Synergy-H1 reader and the fluorescence signal is monitored. Ubiquitination is quantified based on the ratio of acceptor–donor fluorescence (excitation 515 nm; donor emission 570 nm, acceptor emission 670 nm).
Figure 3 provides FRET detection of K48-ubiqutination. The intensity of fluorescence signals is shown across a range of wavelength as indicated. The area representing FRET signals is marked.
In parallel, the reaction is analyzed by gel electrophoresis. For this purpose, an aliquot of final reaction mixture (2μL) is subject to SDS-PAGE (Subheading 3.5), followed by imaging with Typhoon FLA 9500 laser scanner (Subheading 3.6).
Figure 4 provides a fluorescent image of K48 ubiquitination. The positions of the fluorescent Ub input as well as the “di-Ub” product are marked. Omission of reaction components abolished ubiquitination. CC0651, a recently discovered Cdc34 inhibitor [13], is added to the reaction at concentration of 2, 10 or 100μM, resulting in inhibition of the ubiquitination.
Fig. 3.
Detect K48 ubiquitination by FRET. FRET K48 di-Ub assay was carried out as described in Subheading 3.4. The fluorescence signals ranging from 545 to 700 nm were shown. Only the complete reaction yielded FRET
Fig. 4.
Detect K48 ubiquitination by fluorescence imaging. Aliquots of the reaction products of the FRET K48 di-Ub assay was analyzed by SDS-PAGE (Subheading 3.5) and imaging (Subheading 3.6). Shown is an image of fluorescent reaction products detected by a Typhoon 9500 scanner. Only the complete reaction where all components of the assay were present supported di-Ub formation. In addition, the di-Ub ubiquitination was sensitive to the Cdc34 inhibitor CC0651 [13]
3.5. Running the Gel
Remove the gel from the wrapper, remove the comb, and flush the wells with water before placing in the gel apparatus.
Pour running buffer into the chamber and ensure that it covers the wells of the gel.
Boil the samples in SDS loading buffer (1× final concentration) for 5 min at 95 °C and centrifuge briefly afterward to collect the sample.
Load 2–5μL of protein standard, along with your samples, using gel-loading tips.
Connect the power supply and run at 150 V until the loading dye approaches the bottom of the gel (or until the 10 kDa marker approaches the bottom).
3.6. Fluorescence Imaging of the Gel
When the gel is finished running, remove it from the cassette, cut off the wells and bottom of the gel, and place it into PBS in a tray.
Place the gel on a prewetted area in the glass screen within the Typhoon FLA 9500 laser scanner.
Scan the gel.
Acknowledgments
We thank R. Chong, Q. Yu, C. Lee, and other members of the Pan lab for their assistance with protocols. This work was supported by Public Health Service grants CA251425-01 and GM122751-01 to Z-Q P.
Footnotes
The E3 ROC1-CUL1 CTD complex and fluorescently labeled Ub-Q31C/K48R as well as Ub-E64C/ΔGG are not commercially available. While E1 activating enzyme and E2 enzyme Cdc34 can be purchased from Boston Biochem, we provide the protocols for the preparation of E1 and Cdc34 for cost-saving.
pHisTEVyUb-Q31C/K48R leads to bacterial expression of a variant form of Ub bearing Q31C and K48R substitutions. These changes allow the placement of a fluorophore at amino acid position C31 through maleimide linkage (Fig. 1). The resulting fluorescent Ub can only act as a donor in Cdc34-catalyzed ubiquitination due to the lack of K48 (Fig. 2). Additional information can be found in ref. 6.
pHisTEVyUb-E64C/ΔGG leads to bacterial expression of a variant form of Ub bearing E64C substitution and G75G76 deletion. These changes allow the placement of a fluorophore at amino acid position C64 through maleimide linkage (Fig. 2). The resulting fluorescent Ub can only act as a receptor due to the lack of C-terminal G75G76 motif (Fig. 2). Additional information can be found in ref. 6.
pET3a-Uba1 (E1)-His10 is described in Addgene (Plasmid #63571; https://www.addgene.org/63571/).
pET28a-His6-TEV Cdc34 C191S/C223S leads to bacterial expression of a variant form of Cdc34 bearing C191S and C223S substitutions [9]. This E2 variant is indistinguishable from the wild type in reconstituted ubiquitination assays.
pETDuet-1-MCS-I-CUL1 (L421E, V451E, V452K, Y455K)/MCS-II-ROC1 leads to bacterial coexpression of ROC1 and the C-terminal fragment of CUL1 amino acids 411–776. Substitutions L421E, V451E, V452K, Y455K are introduced to improve CUL1 protein solubility [14] without diminishing its activity in reconstituted ubiquitination assays.
Glycerol stocks can be made and stored long term at −80 °C.
Enzymes are stored at −80 °C. Multiple freeze–thaw cycles can decrease the activity of the enzyme, so it is best stored in small aliquots. All other enzymes should also be stored at −80 °C.
For long-term storage, fluorescently labeled Ubs are kept at −80 °C. For everyday use, an aliquot can be stored at 4 °C for a period of 6 months without significant loss of activity in ubiquitination.
It is important to only thaw enzymes immediately before use, and put them away immediately afterward.
The concentrations of all five protein reagents have been determined based on Km measurement.
As shown, di-Ub product of expected size formed only in the complete reaction (Fig. 4). Likewise, a Robust FRET signal is detected only when all five proteins were present (Fig. 3). Thus, the observed FRET truly reflects formation of the K48-linked di-Ub chain as a result of enzymatic synthesis. Note the optional positioning of fluorophore in Ub is critical, as N-terminally labeled Ub, commonly used in previous studies [11, 12], is inactive in our assay. Commercial fluorescent Ub proteins do not specify the locations of the fluorescent dye in Ub and it is unclear if such reagents can be generally optimal for this Ub K48-specific FRET assay.
Z′ factor measures statistical effect size based on the means and standard deviations of both the positive and negative control samples. A Z′ factor value in the range of 0.5–1 is indicative of an excellent assay for high-throughput screening [15]. As detailed in ref. 6, the Z′ factor of the FRET K48 di-Ub assay is 0.86, suggesting that this reporter system provides a sufficient screening window.
The reactions can be stored at −20 °C at this point, and can be run at a later time.
References
- 1.Koepp DM, Harper JW, Elledge SJJW (1999) How the cyclin became a cyclin: regulated proteolysis in the cell cycle. Cell 97(4):431–434 [DOI] [PubMed] [Google Scholar]
- 2.Hershko A, Ciechanover A (1998) The ubiquitin system. Annu Rev Biochem 67:425–479 [DOI] [PubMed] [Google Scholar]
- 3.Petroski MD, Deshaies RJ (2005) Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol 6:9–20 [DOI] [PubMed] [Google Scholar]
- 4.Sarikas A, Hartmann T, Pan ZQ (2011) The cullin protein family. Genome Biol 12(4):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skaar JR, D’Angiolella V, Pagan JK, Pagano M (2009) SnapShot: F box proteins II. Cell 137 (7):1358–1358 e1351 [DOI] [PubMed] [Google Scholar]
- 6.Wu K, Chong RA, Yu Q et al. (2016) Suramin inhibits cullin-RING E3 ubiquitin ligases. Proc Natl Acad Sci U S A 113(14):E2011–E2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu K, Kovacev J, Pan Z-Q (2010) Priming and extending: a UbcH5/Cdc34 E2 handoff mechanism for polyubiquitination on a SCF substrate. Mol Cell 37:784–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong RA, Wu K, Spratt DE et al. (2014) Pivotal role for the ubiquitin Y59-E51 loop in lysine 48 polyubiquitination. Proc Natl Acad Sci U S A 111(23):8434–8439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spratt DE, Wu K, Kovacev J et al. (2012) Selective recruitment of an E2~ubiquitin complex by an E3 ubiquitin ligase. J Biol Chem 287 (21):17374–17385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleiger G, Saha A, Lewis S et al. (2009) Rapid E2-E3 assembly and disassembly enable processive ubiquitylation of cullin-RING ubiquitin ligase substrates. Cell 139(5):957–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madiraju C, Welsh K, Cuddy MP et al. (2012) TR-FRET-based high-throughput screening assay for identification of UBC13 inhibitors. J Biomol Screen 17(2):163–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlson CB, Horton RA, Vogel KW (2009) A toolbox approach to high-throughput TR-FRET-based SUMOylation and DeSUMOylation assays. Assay Drug Dev Technol 7:348–355 [DOI] [PubMed] [Google Scholar]
- 13.Huang H, Ceccarelli DF, Orlicky S et al. (2014) E2 enzyme inhibition by stabilization of a low-affinity interface with ubiquitin. Nat Chem Biol 10(2):156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duda DM, Borg LA, Scott DC et al. (2008) Structural insights into NEDD8 activation of Cullin-RING ligases: conformational control of conjugation. Cell 134(6):995–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J-H, Chung TDY, Oldenburg KR (1999) A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen 4:67–73 [DOI] [PubMed] [Google Scholar]
- 16.Phillips C, Thrower J, Pickart CM, Hill CP (2001) Structure of a new crystal form of tetraubiquitin. Acta Crystallogr D Biol Crystallogr 57(Pt 2):341–344 [DOI] [PubMed] [Google Scholar]