Figure 2. . Recent development of TCIs in the clinic.
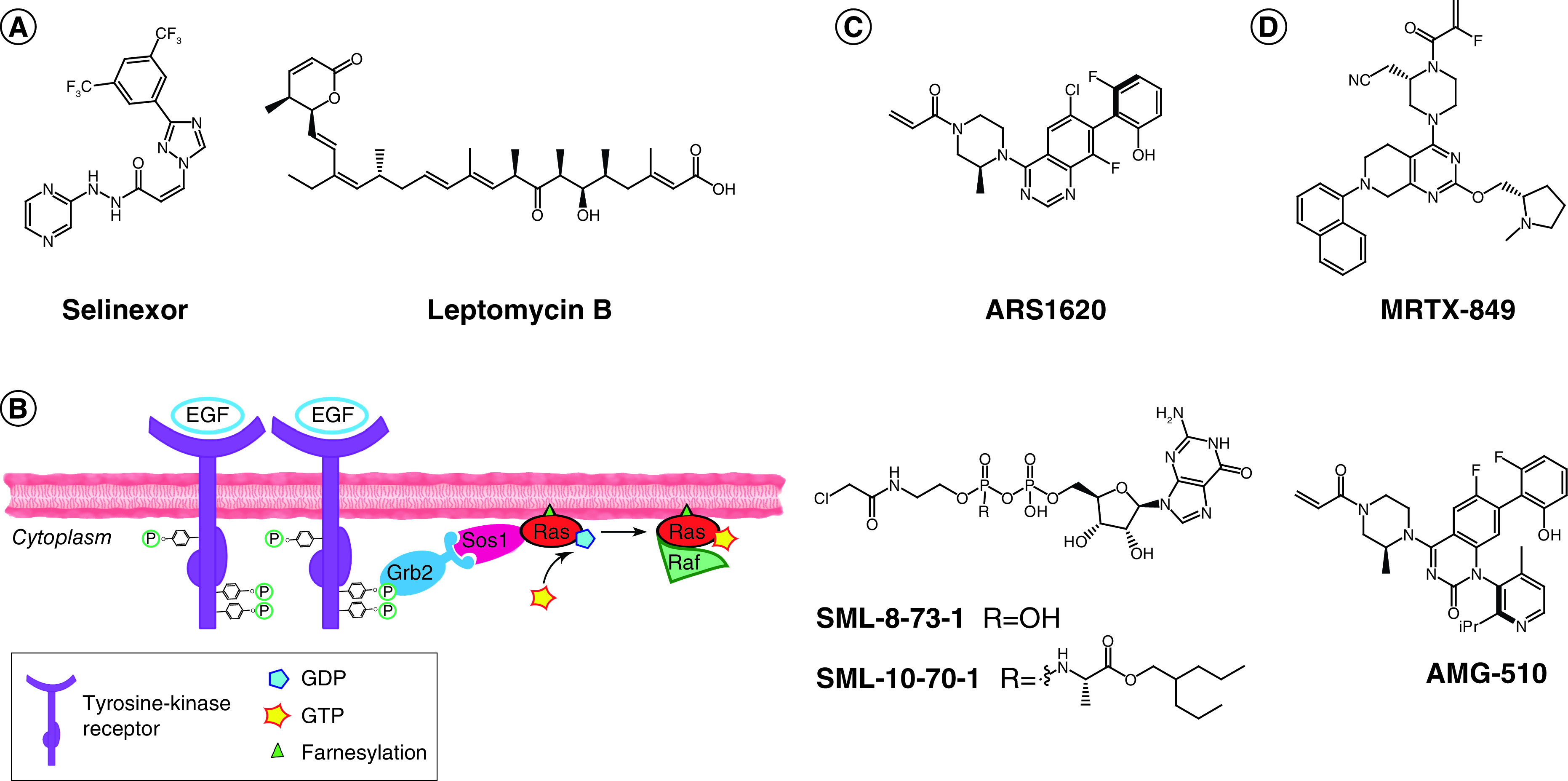
(A) Chemical structures of selinexor (left, FDA approved in 2019) and leptomycin B (right); both compounds bind covalently to Cys528 of XPO-1. (B) Ras activation cascade: upon detection of extracellular signal (e.g., EGF), tyrosine kinase receptors (e.g., EGFR) dimerize and cross-phosphorylate. Phosphate groups are recognized by an adaptor protein (e.g., GRB2), which activates a GEF protein (e.g., SOS1). SOS1 binds to GDP-RAS and induces nucleotide release. Cytoplasmic abundant GTP binds to RAS, resulting in the active conformation for signal transduction. Effector binding (e.g., Raf) occurs and the signal is further propagated to the cytoplasm, ultimately leading to regulation of specific gene expression. (C) Optimized covalent probes for KRASG12C. (D) Clinical candidates against KRASG12C for which the chemical structure is reported in the literature.
