Abstract
Background and Objectives
To clinically, genetically, and histopathologically characterize patients presenting with an unusual combination of distal myopathy and facial weakness, without involvement of upper limb or shoulder girdle muscles.
Methods
Two families with a novel form of actininopathy were identified. Patients had been followed up over 10 years. Their molecular genetic diagnosis was not clear after extensive investigations, including analysis of candidate genes and FSHD1-related D4Z4 repeats.
Results
Patients shared a similar clinical phenotype and a common pattern of muscle involvement. They presented with a very slowly progressive myopathy involving anterior lower leg and facial muscles. Muscle MRI finding showed complete fat replacement of anterolateral compartment muscles of the lower legs with variable involvement of soleus and gastrocnemius but sparing thigh muscles. Muscle biopsy showed internalized nuclei, myofibrillar disorganization, and rimmed vacuoles. High-throughput sequencing identified in each proband a heterozygous single nucleotide deletion (c.2558del and c.2567del) in the last exon of the ACTN2 gene. The deletions are predicted to lead to a novel but unstructured slightly extended C-terminal amino acid sequence.
Discussion
Our findings indicate an unusual form of actininopathy with specific molecular and clinical features. Actininopathy should be considered in the differential diagnosis of distal myopathy combined with facial weakness.
Distal myopathies are genetic muscle diseases, presenting at the onset with weakness of foot and lower leg and/or hand and forearm muscles, which cause progressive loss of muscle tissue.1 Prominent anterior lower leg weakness, facial weakness, and scapular winging are the hallmarks of facioscapulohumeral muscular dystrophy.2 Facial weakness has also been reported in patients with distal myopathies due to mutations in ADSSL1, RYR1, MYH7, NEB, and DNM2.1
We have been following up for many years 2 unrelated patients with an unusual combination of distal lower limb myopathy and facial weakness without involvement of upper limbs or shoulder girdles. Proband 1 (F1,II:4) is a Finnish woman in her 60s (eFigure1, links.lww.com/NXG/A442). The proband's first neurologic examination in 2005 revealed weakness of ankle dorsiflexion and mild atrophy of anterior lower leg muscles. Mild facial weakness, noticeable since her late childhood, was also observed (Table). Weakness slowly progressed over the years. At age 59 years, she displayed severe bilateral foot drop and a moderate, slightly asymmetric facial weakness of frontalis, orbicularis oculi, and perioral muscles without ptosis or external ophthalmoplegia. She did not show scapular winging or any proximal weakness. Echocardiography did not show any cardiac abnormalities. Proband's daughter (F1,III:2), currently in her 30s, had had conservatively treated scoliosis as a teenager. At around age 35 years, she developed mild bilateral foot drop and lower facial weakness with inability to whistle.
Table.
Clinical Data of the Reported Patients
The second proband (F2,II:2) is a 58-year-old Italian man (eFigure1). He had presented with tachyarrhythmia and lower limb distal weakness since his early adulthood (Table). The disease later progressed to proximal lower limb and facial muscles (eFigure2, links.lww.com/NXG/A443) without scapular and upper limbs involvement. He also developed dilated cardiomyopathy.
Lower leg muscle MRI finding showed a similar pattern with complete fatty replacement of anterolateral compartment muscles of the lower legs but largely sparing thigh muscles (Figure 1).
Figure 1. Muscle MRI Findings in the Finnish (A–C) and Italian (D–F) Probands.

(A) Thigh: normal; (B and C) lower leg: anterolateral compartment muscles, gastrocnemius medialis, and distal soleus muscles bilaterally are replaced by fibrofatty tissue. (D) Thigh: mild diffuse fatty degenerative changes in vastus intermedius and hamstring muscles; (E and F) lower leg: anterolateral compartment muscles and bilateral soleus muscles are completely replaced by fibrofatty tissue. Mild changes in gastrocnemius medialis.
The probands did not have any FSHD-1–causing mutation. High-throughput sequencing analysis (for F1,II:4, Nimblegen SeqcapEz Human Exome Library v2.0; Roche, Basel, Switzerland, and for F2,II:1, ClearSeq Inherited DiseaseXT; Agilent Technologies, Santa Clara, CA) identified single nucleotide deletions in the ACTN2 last exon and did not detect causative mutations in SMCHD1 or other myopathy-causing genes. The variant NM001103:c.2567del in the Finnish patients causes a frameshift predicted to replace the last 42 amino acids with 44 novel (p.Pro856Argfs*45) (eFigure3, links.lww.com/NXG/A444). The variant segregated with the disease in the proband's daughter and was absent in the unaffected relatives tested. The ACTN2 deletion, NM001103:c.2558del, identified in the Italian patient, replaces the 45 final amino acids (p.Glu853Glyfs*48) and results in a C-terminal amino acid sequence similar to the one resulting from the c.2567del variant (eFigure3). The variant was not present in the proband's healthy mother and brother.
The identified variants are not listed in gnomADv2.1.1 and are not anticipated to result in nonsense-mediated decay. The variants replace the entire second EF (EF3-4) domain that is needed for alpha-actinin 2 dimerization and for its binding to titin3 (eFigure3).
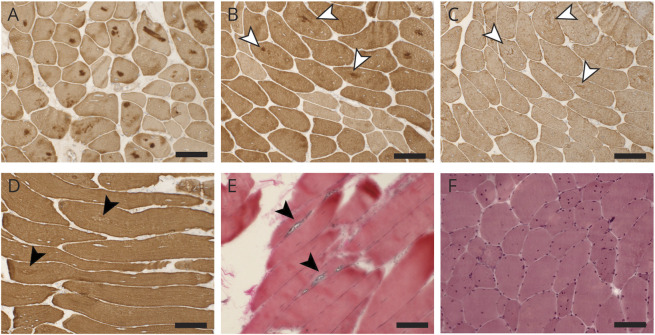
Muscle biopsies showed internalized nuclei and fiber size variation. Immunohistochemical analysis was performed on the Finnish patients using monoclonal antibodies against desmin (Biogenex, Fremont, CA; clone D33), myotilin (Leica Biosystems, Wetzlar, Germany; clone RSO34), alpha-B-crystallin (Novus Biologicals, Littleton, CO; clone 1D11C6E6), and alpha-actinin (Sigma-Aldrich, St. Louis, MO; clone EA-53). In the Finnish patients' biopsies, there were rimmed vacuoles and myofibrillar aggregates, strongly positive for alpha-crystallin and myotilin and weakly positive for desmin (Figure 2). Nicotinamide adenine dinucleotide stain showed core-like pathology.
Figure 2. Histopathology.
Immunohistochemical stainings of the muscle biopsy of the Finnish proband (A–D) show central myofibrillar aggregations in several fibers positive for alpha-B-crystallin (A) and myotilin (B), whereas desmin (C) shows weak positivity; B and C are serial sections, and the same fibers are indicated with white arrowheads. Alpha-actinin staining (D) shows areas of myofibrillar disorganization, indicated by arrowheads. Herovici staining (E) of the muscle biopsy of the daughter of the Finnish proband shows 2 fibers with prominent rimmed vacuoles (arrowheads). Hematoxylin and eosin staining of the muscle biopsy of the Italian proband (F) shows fiber size variation and multiple internal nuclei. Scale bar = 100 μm.
In the Finnish proband's muscle biopsy, immunochemistry showed minor irregular staining of alpha-actinin pinpointing the areas of myofibrillar disarray (Figure 2), which, however, could simply reflect disorganization of the underlying myofibrils. No clear accumulation of alpha-actinin was observed (the antibody recognizes both alpha-actinin 2 and 3, but the patient has no expression of alpha-actinin 3, being homozygous for the ACTN3 p.R577X variant4). A transcriptome analysis (library prepared using the NEBNext Ultra II Directional RNA library Prep for Illumina, New England Biolabs) on the same sample confirms that the variant c.2567del results in a normal, biallelic expression of ACTN2 transcripts.
In 2019, we described a distal myopathy, without facial weakness, caused by ACTN2 missense variants in 4 families.5 De novo ACTN2 variants were identified in 2 patients with congenital myopathy with structured cores, showing mild facial weakness.6 Missense variants have also been associated with cardiomyopathies7 (eFigure3c, links.lww.com/NXG/A444).
In this study, we describe a novel form of dominant distal actininopathy to be considered in the differential diagnosis of patients having lower leg predominant distal myopathy with facial weakness.
Standard Protocol Approvals and Patient Consents
Patients provided informed consent. Ethical approval falls under HUS:195/13/03/00/11.
Data Availability
Deidentified data are available on request.
Acknowledgment
The authors acknowledge Merja Soininen for technical assistance, Meharji Arumilli and the TIGEM bioinformatics core for bioinformatics assistance, and CSC-IT Center for Science Ltd. for providing computational resources. Sequencing for patient 1 was performed by the Sequencing unit of Institute for Molecular Medicine Finland FIMM Technology Centre, University of Helsinki, which is supported by Biocenter Finland. Sequencing for patient 2 was performed by the TIGEM Next Generation Sequencing facility.
Appendix. Authors

Contributor Information
Marco Savarese, Email: marco.savarese@helsinki.fi.
Anna Vihola, Email: anna.vihola@helsinki.fi.
Sanna Pauliina Huovinen, Email: sanna.huovinen@fimlab.fi.
Simonetta Gerevini, Email: sgerevini@asst-pg23.it.
Annalaura Torella, Email: annalaura.torella@gmail.com.
Mridul Johari, Email: mridul.johari@helsinki.fi.
Marina Scarlato, Email: scarlato.marina@hsr.it.
Per Harald Jonson, Email: per-harald.jonson@helsinki.fi.
Maria Elena Onore, Email: mariaelena.onore@unicampania.it.
Peter Hackman, Email: peter.hackman@helsinki.fi.
Mathias Gautel, Email: mathias.gautel@kcl.ac.uk.
Vincenzo Nigro, Email: nigro@tigem.it.
Stefano Carlo Previtali, Email: previtali.stefano@hsr.it.
Bjarne Udd, Email: bjarne.udd@netikka.fi.
Study Funding
Clinical and genetic data of the index patient were shared in RD‐Connect, which received funding from the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 305444 within the Solve-RD project. The solve-RD project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement no. 779257. M. Savarese was supported by grants from AFM-Telethon, Sydäntutkimussäätiö, and Paulon Säätiö. M. Gautel was supported by a grant from Wellcome Trust (Collaborative Award in Sciences 201543/Z/16/Z). M. Gautel holds the BHF Chair of Molecular Cardiology.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/NG for full disclosures.
References
- 1.Savarese M, Sarparanta J, Vihola A, et al. Panorama of the distal myopathies. Acta Myol. 2020;39(4):245-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sacconi S, Briand-Suleau A, Gros M, et al. FSHD1 and FSHD2 form a disease continuum. Neurology. 2019;92(19):E2273-E2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson RA, Joseph C, Kelly G, et al. Ca2+-independent binding of an EF-hand domain to a novel motif in the alpha-actinin-titin complex. Nat Struct Biol. 2001;8(10):853-857. [DOI] [PubMed] [Google Scholar]
- 4.North KN, Yang N, Wattanasirichaigoon D, Mills M, Easteal S, Beggs AH. A common nonsense mutation results in alpha-actinin-3 deficiency in the general population. Nat Genet. 1999;21(4):353-354. [DOI] [PubMed] [Google Scholar]
- 5.Savarese M, Palmio J, Poza JJ, et al. Actininopathy: a new muscular dystrophy caused by ACTN2 dominant mutations. Ann Neurol. 2019;85(6):899-906. [DOI] [PubMed] [Google Scholar]
- 6.Lornage X, Romero NB, Grosgogeat CA, et al. ACTN2 mutations cause “multiple structured core disease” (MsCD). Acta Neuropathol. 2019;137(3):501-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu C, Bagnall RD, Ingles J, et al. Mutations in alpha-actinin-2 cause hypertrophic cardiomyopathy: a genome-wide analysis. J Am Coll Cardiol. 2010;55(11):1127-1135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified data are available on request.