Optimization of reaction conditionsa.
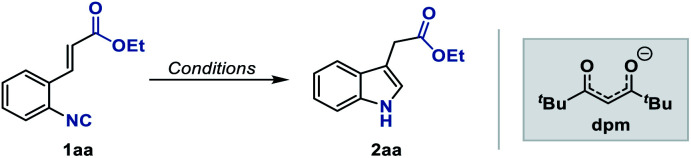
| ||||
|---|---|---|---|---|
| Entry | Catalyst | Solvent | Reductant | Yield (%) |
| 1b | Fe(acac)3 | i-PrOH | PhSiH3 | 25 |
| 2b | Fe(dibm)3 | i-PrOH | PhSiH3 | 59 |
| 3b | Fe(dpm)3 | i-PrOH | PhSiH3 | 75 |
| 4 | Fe(dpm)3 | i-PrOH | PhSiH3 | 82 |
| 5b | Co(dpm)3 | i-PrOH | PhSiH3 | 21 |
| 6b | Mn(dpm)3 | i-PrOH | PhSiH3 | 46 |
| 7b | — | i-PrOH | PhSiH3 | NR |
| 8 | Fe(dpm)3 | EtOH | PhSiH3 | 56 |
| 9 | Fe(dpm)3 | t-BuOH | PhSiH3 | 77 |
| 10 | Fe(dpm)3 | DCE | PhSiH3 | NR |
| 11 | Fe(dpm)3 | i-PrOH/DCE | PhSiH3 | 82 (81)c |
| 12 | Fe(dpm)3 | i-PrOH | Et3SiH | 12 |
| 13 | Fe(dpm)3 | i-PrOH | (EtO)3SiH | 28 |
| 14d | Fe(dpm)3 | i-PrOH | DEMS | 81 |
Reaction conditions: 1aa (0.2 mmol), catalyst (5 mol%), hydrosilane (0.4 mmol), solvent (1.2 mL), 35 °C, 2 h. Yield determined by GC using n-hexadecane as the internal standard.
Used 30 mol% catalyst.
Isolated yield.
Reaction at 60 °C for 5 h. acac = acetylacetone, dibm = diisobutyrylmethane, dpm = dipivaloylmethane.
