Abstract
Objective
To examine the clinicopathologic factors that contribute to regional and distant recurrence in intermediate to high risk head and neck melanoma patients after sentinel lymph node biopsy (SLNB).
Methods
This study is a retrospective review from an academic tertiary care center. Patients treated with SLNB for head and neck melanoma from 1997 to 2019 were reviewed and characterized by sentinel lymph node (SLN) status. Clinical variables were examined for the impact on regional and distant recurrence in SLNB‐negative patients using univariable and multivariable Cox regression analysis.
Results
One hundred and fifty four patients were included. Of note, 127 (82.5 %) were men, and the average age was 61.3 years. Median follow‐up was 68.6 weeks. Pathologic review of SLNs found 3.9% positive for metastatic melanoma; 96.1% were negative. Regional recurrence was significantly associated with tumor stage and age on multivariate analysis. A total of 4.5% of patients recurred in a previously labeled negative basin. Scalp subsite accounted for 30.5% of primary tumors and was more likely to yield a positive SLN on univariate analysis (P = .023). Tumor stage and age were significantly associated with distant metastasis on multivariable analysis (P = .026, P < .001 respectively).
Conclusion
We report a number of prognostic trends in head and neck melanoma. SLN positivity was found more often in patients with a primary tumor of the scalp. Regional recurrence was significantly associated with age and tumor stage, whereas distant recurrence was significantly associated with tumor staging and scalp subsite. Scalp subsite was associated with an increased risk for nodal metastasis and distant recurrence.
Level of evidence
3.
Keywords: head and neck cancer, melanoma, sentinel lymph node
1. INTRODUCTION
First described by Morton et al in 1992, sentinel lymph node biopsy (SLNB) has revolutionized the management of melanoma by helping to further evaluate the status of regional lymph nodes through histopathologic analysis.1 When positive, SLNB predicts the status of the regional lymph node basin and helps identify high‐risk patients who might benefit from completion lymphadenectomy or adjuvant therapy.2, 3 Several studies have illustrated that patients with positive sentinel lymph node (SLN) histopathology have an improved melanoma specific survival compared to patients who develop clinically positive nodes at a later time.1, 2, 4, 5 This is critical, since as many as 15% to 20% of patients with cutaneous melanoma of the head and neck (CMHN) without cervical lymphadenopathy at presentation can go on to develop regional metastases after primary resection.6
When compared to the trunk and extremities, surgical management of regional melanoma metastases in the head and neck poses unique challenges due to both the complex lymphatic drainage patterns and increased site‐specific morbidity associated with lymphadenectomy.2, 7 Further, although the utility of SLNB is well documented in the head and neck, concerns regarding its accuracy in this region are debated due to reports of both low positivity rate and high recurrence rates in previously mapped negative nodal basins.3, 8, 9, 10 Taken together, this information suggests potentially inferior accuracy of SLNB in the head and neck when compared to the trunk and extremities, which has been reported by several series.11, 12, 13 To add to the existing body of literature, we aimed to identify risks for false negative SLNB as well as prognostic trends and outcomes after SLNB for CMHN. We report results from a cohort of patients treated at a single tertiary medical institution over a 22‐year period.
2. MATERIALS AND METHODS
This retrospective review was approved by the University of California, Los Angeles (UCLA) Institutional Review Board. From January 1997 to July 2019, patients diagnosed with CMHN who underwent SLNB were identified by a computer‐assisted search performed by the UCLA Tumor Registry. Clinicopathologic data collected included tumor characteristics, diagnostic information, treatment modalities, and patient outcomes. Clinical staging was determined according to the American Joint Committee on Cancer staging system, eighth edition.14 Primary tumor characteristics were evaluated including location, Breslow thickness, Clark's level, mitotic rate, ulceration status, perineural, and lymphovascular invasion.
All patients underwent SLNB using lymphoscintography with a technetium labeled colloid injected at the primary site. Intraoperatively, intradermal blue dye was injected into the primary site and used in conjunction with a gamma probe to help identify the SLN. A lymph node that assessed ≥10% of the highest emitting node was considered a SLN.
Results of SLNB were recorded from operative and histopathologic reports performed by pathologists. SLNs were prepared into multiple serial permanent sections and stained with hematoxylin and eosin for histopathological assessment. Additional immunohistochemistry was performed at the discretion of the pathologist, which included S‐100 and HMB‐45. Patient records were reviewed for recurrence after completion of SLNB. Those patients that had a recurrence in a previously “negative” SLN basin were characterized as “false negatives”.15 Recurrences were further characterized as regional or distant.
Descriptive statistics were compiled for the overall sample and stratified by nodal disease (negative vs positive) as well as SLNB negative status (true vs false negative). The independent‐samples t test was used to compare differences between group means, and Fisher's exact test was used to examine group differences in categorical variables.
The primary outcome was recurrence‐free survival (RFS). Follow‐up duration was calculated from the date of SLNB to the date of death or last reported follow up. Risk of regional recurrence and risk of distant recurrence were assessed in separate survival analyses for patients with negative SLNB. When analyzing regional recurrence, patients with local and distant recurrence were censored at the time of local and distant recurrence, respectively. When analyzing distant recurrence, patients with local and regional recurrence were censored at their respective times of recurrence. Kaplan‐Meier survival curves were constructed based on the recurrence data. Associations between demographic and clinicopathologic variables and recurrence were examined using univariable Cox regression analysis. Multivariable Cox regression was performed using variables with P < .1 on univariable analysis, with the exception of Breslow thickness and Clark's level that were excluded from multivariable analysis due to collinearity with tumor stage. All statistical analyses were carried out using the R statistical computing environment version 3.6.1. P values <.05 were considered statistically significant.
3. RESULTS
Between January 1997 to August 2019, 243 patients were entered into the UCLA Tumor Registry as having undergone SLNB. Of these patients, 154 patients were included who underwent a SLNB for CMHN. Patients with non‐head and neck subsites, non‐melanoma pathology, insufficient records, incomplete treatment, or cases of recurrent melanoma were excluded. Demographic and clinicopathologic characteristics are described in Table 1. In this patient population, the mean age at diagnosis was 61.3 years and the majority of patients were male (82.5%). The majority of cases were located on the face (38.3%), followed by the scalp (30.5%), ear (17.5%), and neck (13.6%). The average Breslow depth was 1.9 mm (0.2‐10.0). Ulceration was most often absent from surgical specimens (81 patients, 63.8%). Tumors were most frequently characterized as T2 (40.1%), whereas a minority were T4 (10.6%). On histopathologic analysis, six SLNBs (3.9%) were positive for metastatic carcinoma. The mean number of nodes submitted from a SLNB was 2.1.
TABLE 1.
Demographic and clinicopathologic characteristics stratified by nodal disease status and SLNB negative statusa
| Overall (N = 154) | Nodal disease | P value | SLNB negative status | P value | |||
|---|---|---|---|---|---|---|---|
| Negative (n = 141) | Positive (n = 13) | True (n = 141) | False (n = 7) | ||||
| Age, mean (SD) | 61.3 (14.9) | 61.0 (14.9) | 64.9 (14.5) | .365 | 61.0 (14.9) | 71.0 (11.0) | .054 |
| Sex, N (%) | .701 | .607 | |||||
| Male | 127 (82.5) | 117 (83.0) | 10 (76.9) | 117 (83.0) | 5 (71.4) | ||
| Female | 27 (17.5) | 24 (17.0) | 3 (23.1) | 24 (17.0) | 2 (28.6) | ||
| Breslow thickness, mean (SD) | 1.9 (1.6) | 1.8 (1.6) | 2.4 (1.8) | .268 | 1.8 (1.5) | 2.2 (1.5) | .592 |
| T stage, N (%) | .170 | .519 | |||||
| T1 | 43 (30.3) | 42 (32.3) | 1 (8.3) | 42 (32.3) | 1 (16.7) | ||
| T2 | 57 (40.1) | 52 (40.0) | 5 (41.7) | 52 (40.0) | 2 (33.3) | ||
| T3 | 27 (19.0) | 23 (17.7) | 4 (33.3) | 23 (17.7) | 2 (33.3) | ||
| T4 | 15 (10.6) | 13 (10.0) | 2 (16.7) | 13 (10.0) | 1 (16.7) | ||
| Clark's level, N (%) | .148 | .397 | |||||
| ≤3 | 40 (38.8) | 39 (41.5) | 1 (11.1) | 39 (41.5) | 1 (16.7) | ||
| >3 | 63 (61.2) | 55 (58.5) | 8 (88.9) | 55 (58.5) | 5 (83.3) | ||
| Ulceration Bx, N (%) | .722 | 1 | |||||
| Absent | 81 (63.8) | 76 (64.4) | 5 (55.6) | 76 (64.4) | 3 (60.0) | ||
| Present | 46 (36.2) | 42 (35.6) | 4 (44.4) | 42 (35.6) | 2 (40.0) | ||
| Mitotic rate, N (%) | .481 | 1 | |||||
| <1 | 49 (39.2) | 47 (40.5) | 2 (22.2) | 47 (40.5) | 2 (50.0) | ||
| ≥1 | 76 (60.8) | 69 (59.5) | 7 (77.8) | 69 (59.5) | 2 (50.0) | ||
| LVI, N (%) | 1 | 1 | |||||
| Absent | 112 (92.6) | 104 (92.0) | 8 (100.0) | 104 (92.0) | 4 (100.0) | ||
| Present | 9 (7.4) | 9 (8.0) | 0 (0.0) | 9 (8.0) | 0 (0.0) | ||
| PNI, N (%) | 1 | 1 | |||||
| Absent | 113 (93.4) | 105 (92.9) | 8 (100.0) | 105 (92.9) | 4 (100.0) | ||
| Present | 8 (6.6) | 8 (7.1) | 0 (0.0) | 8 (7.1) | 0 (0.0) | ||
| Subsite, N (%) | .023 | .194 | |||||
| Non‐scalp | 107 (69.5) | 102 (72.3) | 5 (38.5) | 102 (72.3) | 3 (42.9) | ||
| Scalp | 47 (30.5) | 39 (27.7) | 8 (61.5) | 39 (27.7) | 4 (57.1) | ||
| Number of SLNs, N (%) | .571 | 1 | |||||
| 1 | 84 (54.5) | 78 (55.3) | 6 (46.2) | 78 (55.3) | 4 (57.1) | ||
| >1 | 70 (45.5) | 63 (44.7) | 7 (53.8) | 63 (44.7) | 3 (42.9) | ||
| SLN status, N (%) | ‐ | ‐ | |||||
| True positive | 6 (3.9) | 0 (0.0) | 6 (46.2) | 0 (0.0) | 0 (0.0) | ||
| True negative | 141 (91.6) | 141 (100.0) | 0 (0.0) | 141 (100.0) | 0 (0.0) | ||
| False negative | 7 (4.5) | 0 (0.0) | 7 (53.8) | 0 (0.0) | 7 (100.0) | ||
Abbreviations: LVI, lymphovascular invasion; PNI, perineural invasion; SLN, sentinel lymph node; SLNB, sentinel lymph node biopsy.
Boldface indicates P < .05.
Median follow up for all patients was 68.6 weeks and the average time to recurrence was 109.9 weeks. Of all patients, 13 (8.4%) recurred regionally and 22 (14.3%) distantly. Of the patients that recurred regionally, two had a prior positive SLNB and 11 had a prior negative SLNB. Retrospective results for SLNB status were determined as follows: true positive in 6 cases (3.9%), true negative in 141 cases (91.6%), and false negative in 7 cases (4.5%). To further elucidate the cause of our low true positive rate, T1 patients were excluded from the analysis; when this was done, the number was found to rise to 6.1%. This calculation was in accordance with prior SLNB papers that exclude T1 tumors.16, 17, 18 Although papers exist that include T1 tumors, no SLN positivity was found in T1 patients.19 False negatives were composed of patients who recurred in the prior negative SLNB basin. The false negative rate (FNR) was calculated by (FN/FN + TP) and was found to be 53.8%. The false negative omission rate was calculated by (FN/[FN + TN]) and was found to be 4.7%. Four of the seven cases of false negative SLNB were scalp primaries (57.1%). Of the six patients with a true positive SLNB, 66.6% had primary disease of the scalp. Five of these patients underwent a completion neck dissection whereas one patient elected to not proceed with neck dissection; none of these patients had additional metastatic melanoma deposits recovered on completion lymphadenectomy. Over the study time frame, 10.4% of patients were documented to have died of any cause. Twelve percent of patients went on to receive radiation, whereas 29% received target therapy/immunotherapy. Higher Clark's level and T stage were significantly associated with patients receiving immunotherapy/targeted therapy (P = .037, P < .001, respectively; Tables S1 and S2).
When stratified by SLNB, tumor subsite was shown to be significantly associated with risk of positive SLNB, as shown in Table 1. Patients with a scalp primary were more likely to have positive SLNB (61.5%), whereas those with non‐scalp primary tumors had negative SLNB (72.3%) (P = .02). The average Breslow depth for patients with scalp primary tumors was 1.86 compared to 1.88 for non‐scalp primary tumors (P = .74). When stratifying patients by true negative and false negative SLNB, the resulting groups did not differ significantly on demographic or clinicopathologic attributes.
Figures 1 and 2 display Kaplan‐Meier curves for regional and distant recurrence, respectively, in patients with negative SLNB. Five‐year RFS was 88.7% (95% CI, 82.3‐95.6) for regional recurrence and 73.3% (95% CI, 62.8‐85.4) for distant recurrence. Univariable and multivariable Cox regression analyses were conducted to evaluate the risk of regional and distant recurrence in patients with negative SLNB. On univariable analysis, age (HR 1.05; 95% CI, 1.00‐1.11), Breslow thickness (HR 1.42, 95% CI, 1.15‐1.75), T4 stage (HR 18.37, 95% CI, 2.14‐157.53), and perineural invasion (HR 8.64; 95% CI, 1.71‐43.50) were associated with risk of regional recurrence as shown in Table 2. On multivariable analysis, patients with T4 stage tumors were significantly more likely to recur regionally than patients with T1 stage tumors (HR 28.28, 95% CI, 2.72‐294.05).
FIGURE 1.
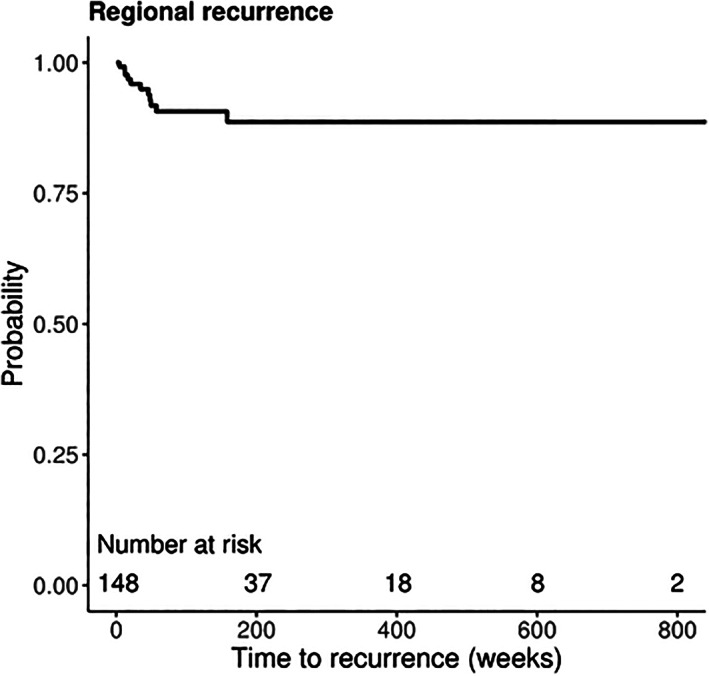
Kaplan‐Meier curve for regional recurrence‐free survival of patients with negative SLNB
FIGURE 2.
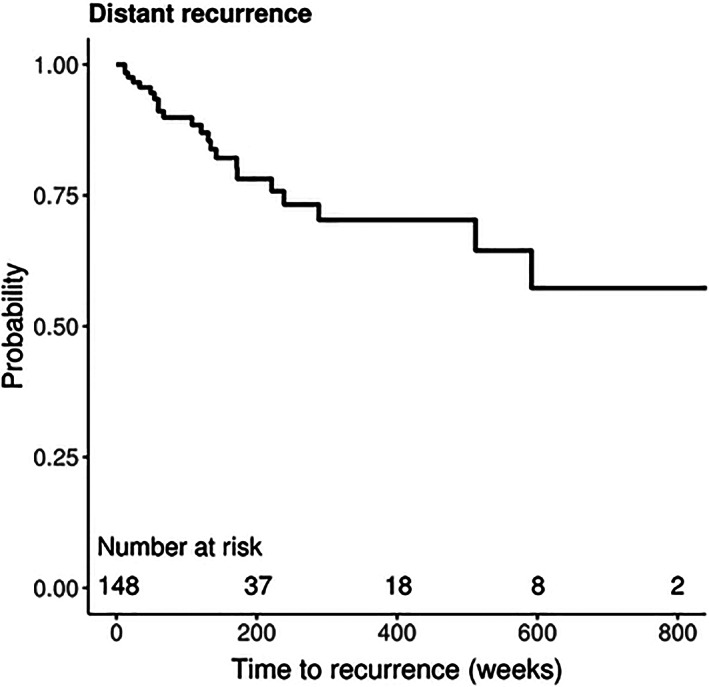
Kaplan‐Meier curve for distant recurrence‐free survival of patients with negative SLNB
TABLE 2.
Cox regression analysis for regional recurrence in patients with negative SLNBa
| Covariates | Univariable | Multivariable (n = 116) | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.05 (1.00‐1.11) | .044 | 1.08 (1.02‐1.16) | .013 |
| Gender | ||||
| Male | Reference | |||
| Female | 0.88 (0.19‐4.05) | .865 | ‐ | |
| Breslow thickness | 1.42 (1.15‐1.75) | .001 | ‐ | |
| Stage | ||||
| T1 | Reference | Reference | ||
| T2 | 1.77 (0.16‐19.49) | .642 | 0.00 (0.00‐Inf) | .998 |
| T3 | 3.64 (0.33‐40.19) | .292 | 3.23 (0.28‐36.82) | .344 |
| T4 | 18.37 (2.14‐157.53) | .008 | 28.28 (2.72‐294.05) | .005 |
| Clark's level | ||||
| ≤3 | Reference | |||
| >3 | 5.92 (0.74‐47.38) | .094 | ‐ | |
| Ulceration | ||||
| Absent | Reference | |||
| Present | 1.74 (0.43‐6.97) | .434 | ‐ | |
| Mitotic rate | ||||
| <1 | Reference | |||
| ≥1 | 0.87 (0.20‐3.91) | .861 | ‐ | |
| LVI | ||||
| Absent | Reference | |||
| Present | 2.25 (0.27‐18.73) | .454 | ‐ | |
| PNI | ||||
| Absent | Reference | Reference | ||
| Present | 8.64 (1.71‐43.50) | .009 | 0.93 (0.17‐5.08) | .931 |
| Subsite | ||||
| Non‐scalp | Reference | |||
| Scalp | 2.13 (0.65‐6.99) | .213 | ‐ | |
| Number of SLNs | ||||
| 1 | Reference | |||
| >1 | 0.79 (0.23‐2.71) | .710 | ‐ | |
Abbreviations: LVI, lymphovascular invasion; PNI, perineural invasion.
Boldface indicates P < .05.
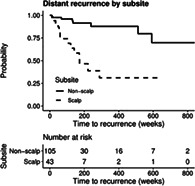
When analyzing distant recurrence (Table 3), patient and tumor characteristics associated with increased risk of recurrence included Breslow thickness (HR: 1.5; 95% CI, 1.25‐1.80), T4 tumor stage (HR: 12.63, 95% CI, 3.21‐49.61), Clark's level (HR: 6.52; 95% CI, 1.44‐29.62), tumor ulceration (HR: 3.79, 95% CI, 1.59‐9.06), and mitotic rate (HR: 4.87, 95% CI, 1.43‐16.64). In addition, primary tumors originating from the scalp showed a significantly elevated risk of distant recurrence (HR: 6.19; 95% CI, 2.56‐14.99). Risk of distant recurrence in patients with non‐scalp compared to scalp primary tumors is shown in Figure 3. On multivariate analysis, T4 tumor stage (HR, 5.06, 95% CI, 1.21‐21.11) and scalp subsite (HR, 6.49, 95% CI, 2.36‐17.81) retained significance as predictors of distant recurrence. Patients who received targeted/immunotherapy had a significantly greater risk of regional and distant recurrence than patients who did not receive targeted therapy (P = .003 and P < .001, respectively). Additionally, patients who received radiation were found to have a significantly greater risk of distant recurrence (P = .008; Table S3 ).
TABLE 3.
Cox regression analysis for distant recurrence in patients with negative SLNBa
| Covariates | Univariable | Multivariable (n = 116) | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.02 (0.99‐1.05) | .204 | ‐ | |
| Gender | ||||
| Male | Reference | |||
| Female | 0.44 (0.10‐1.90) | .274 | ‐ | |
| Breslow thickness | 1.50 (1.25‐1.80) | <.001 | ‐ | |
| Stage | ||||
| T1 | Reference | Reference | ||
| T2 | 1.61 (0.42‐6.20) | .485 | 0.80 (0.21‐3.08) | .741 |
| T3 | 5.84 (1.59‐21.44) | .008 | 1.74 (0.44‐6.88) | .431 |
| T4 | 12.63 (3.21‐49.61) | <.001 | 5.06 (1.21‐21.11) | .026 |
| Clark's level | ||||
| ≤3 | Reference | |||
| >3 | 6.52 (1.44‐29.62) | .015 | ‐ | |
| Ulceration | ||||
| Absent | Reference | Reference | ||
| Present | 3.79 (1.59‐9.06) | .003 | 1.74 (0.63‐4.84) | .288 |
| Mitotic rate | ||||
| <1 | Reference | Reference | ||
| ≥1 | 4.87 (1.43‐16.64) | .011 | 3.60 (0.89‐14.58) | .073 |
| LVI | ||||
| Absent | Reference | |||
| Present | 2.07 (0.47‐9.12) | .336 | ‐ | |
| PNI | ||||
| Absent | Reference | Reference | ||
| Present | 2.97 (0.68‐13.01) | .149 | 0.93 (0.17‐5.08) | .931 |
| Subsite | ||||
| Non‐scalp | Reference | Reference | ||
| Scalp | 6.19 (2.56‐14.99) | <.001 | 6.49 (2.36‐17.81) | <.001 |
| Number of SLNs | ||||
| 1 | Reference | |||
| >1 | 0.54 (0.21‐1.39) | .204 | ‐ | |
Abbreviations: LVI, lymphovascular invasion; PNI, perineural invasion.
Boldface indicates P < .05.
FIGURE 3.
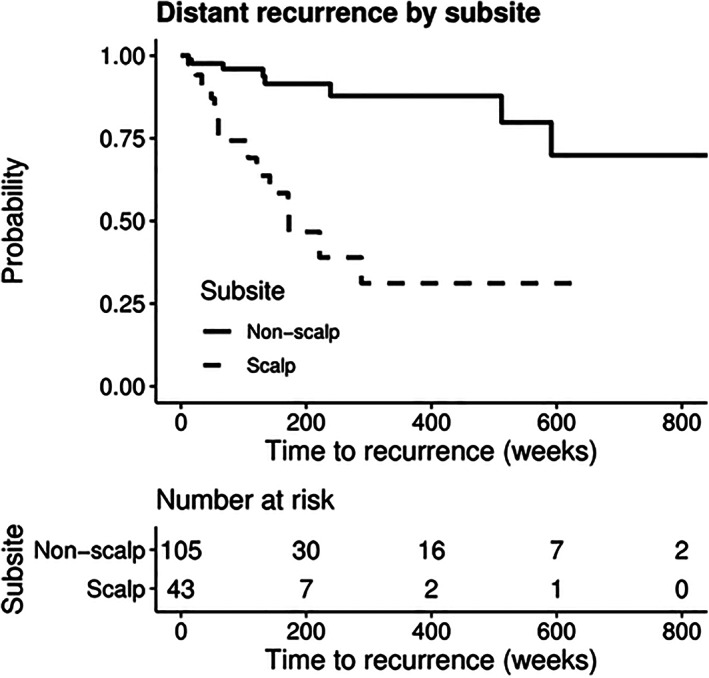
Kaplan‐Meier curve for distant recurrence‐free survival of patients with negative SLNB stratified by subsite
4. DISCUSSION
SLNB has transformed the treatment of melanoma and is a critical part of management for intermediate thickness tumors. The presence of a positive SLN in a patient with clinically negative nodal disease has been identified as one of the most important prognostic predictors of disease free survival.4, 10, 20, 21 Despite the widespread use of SLNB, the head and neck region continues to be described as having a high risk of recurrence in “negative” SLN basins, with subsite of head and neck alone predictive of recurrence.2, 22, 23, 24, 25
Patient characteristics that increase the risk of melanoma are well known, and include Fitzpatrick type I or II,26 advanced age, male gender, and immunosuppressed state.27 Melanoma traditionally affects males at almost twice the rate of women and most often occurs on sun‐exposed regions of the body.28 The demographic makeup of our study is congruent with those previously reported concerning advanced age and preponderance of males.16, 20, 22, 29 The majority of patients included in this analysis were found to have an increased mitotic rate, T stage >T1, and Clark's level > 3.
When performed in the head and neck, SLNB has been shown to have a lower positivity rate when compared to the trunk and extremities.3, 12 A recent study by Erman et al compiled published datasets from SLNB in CMHN and showed a low compiled positivity rate of 13.4%, which is on the lower end of the usual 9.4% to 27% reported in the literature for CMHN patients with positive nodes.2, 9, 30, 31, 32, 33, 34, 35, 36 The authors hypothesize this result is attributable to the highly variable lymphatic drainage of the cervical nodal basins.3 Our study was in concordance with the literature that states that positivity is low in the head and neck, with only 3.9% of SLNs found to contain metastatic melanoma deposits and be “positive.” This low rate is likely multifactorial and may be due to the inclusion of thin (T1) tumors in this cohort. Although many patients had tumors with T2 intermediate thickness (1.1‐2.0 mm; 40.1%), 30.3% of patients presented with T1 tumors with concerning features warranting evaluation by SLNB. When these patients were excluded from the study and positivity is examined, our positivity rate increased to 6.1%. As a result, the inclusion of these patients is likely a large component of our lower positivity rate.
The utility of SLNB in the literature is debated. In a recent study by Main et al, the false positive rate of SLNB was found to be 24%.4 Additional concerns exist, including the idea that micrometastases detected by SLNB would otherwise be cleared by the immune system, as well as a continued increasing mortality from melanoma despite the widespread use of SLNB screening and, therefore, unclear survival benefit.4 The present study identified positive LNs in 3.8% of patients undergoing SLNB. Completion lymphadenectomy was performed in five of the six patients and yielded no further metastatic melanoma deposits. This argues toward SLNB detecting micrometastatic disease, and, therefore, the survival benefit in these patients is unclear.
Predictors for positive SLNB are discussed in the literature, with a major determinant being scalp subsite. Primary melanomas of the scalp represent a unique subset of patients with more aggressive disease and a worsened prognosis when compared to their non‐scalp CMHN counterparts.37 This may be due to presentation with more advanced disease, greater Breslow depths, increased SLN positivity, and lymph node recurrence.16, 38, 39 A recent study by Cappello et al identified scalp SLN positivity to be 20.9% and found this feature to the strongest predictor of overall survival.27 In our series, the scalp subsite accounted for 30.5% of all subjects when comparing patients with a positive SLNB to those with a true negative SNLB; scalp subsite was the only clinicopathologic factor with a significantly increased risk of positive findings on SLN.
False negative rates (FNRs) for HN melanoma SLNB span 3.4% to 32.1% in the literature.9, 20, 22, 23, 24 This has been attributed to many factors, including poor radiotracer technique, failure to localize the SLN, inability to identify microscopic disease, and the complex nodal drainage of the head and neck region.10 Further, the reporting of FNRs is inconsistent in the literature. Some authors report a FNR that describes any recurrence compared to recurrence in the originally negative nodal basin (false negative/false negative + true positive) whereas others report a false omission rate (false negative/false negative + true negative). When calculating our FNR using the aforementioned definition, our rate was found to be 53.8% and is influenced by our low true positive rate (TPR). This number may be “high” compared to the reported range due to inconsistent reporting by prior authors concerning FNR and use of different false negative definitions. Erman et al similarly detail this inconsistency and claim the false omission rate to provide greater clinical value as it represents the proportion of patients who recur in a basin that was originally negative.3 When using this definition, our false omission rate was found to be 4.7%, which is lower than the compiled false omission rate of 9.3% that is quoted in the literature.3
When found in the head and neck, melanoma has a poor prognosis and higher rate of recurrence compared to other parts of the body. Studies have shown a substantial impact on survival in patients who recur after a SLNB compared to those that do not, with as much as a 30% survival decrease.2, 10 Therefore, it is prudent to understand who is at risk of recurrence following a negative SLNB. In our study, age and advanced T stage were found to be significantly associated with a greater risk of regional recurrence despite negative SLNB. This is in agreement with the literature, which reports Breslow thickness and the presence of ulceration as independent predictors of worse overall survival, disease free survival, regional and distant recurrence.4 Furthermore, advanced age has been associated with greater Breslow thickness and was an independent risk factor for recurrence.40 Although increased Breslow has been noted in the literature to contribute to increased aggressive tumor pathology, on further analysis of our scalp vs nonscalp primary tumors, we failed to find a significant difference between average Breslow depths. Further studies are needed to investigate the underlying reason for increased distant metastasis in patients with primary tumors of the scalp.
Patients with negative SLNB have been shown in the literature to have a preponderance for distant first site recurrence in melanoma, often skipping the lymphogenic metastatic route. The reason for this is unclear, but has been hypothesized as occurring due to direct hematogenous routes and more aggressive tumor biology.19, 20, 21 A recent study by Faut et al described 58.5% of patients with negative SLNB to have distant metastases as the first site of recurrence.19 In our series, distant recurrence was the most common first recurrence site, and was found almost exclusively without the presence of concurrent local or regional recurrence. When examining clinicopathologic characteristics that portend risk of distant recurrence, our study identified T4 stage and scalp subsite to contribute to increased risk of distant recurrence. Several studies have reported that scalp melanoma portends a poorer prognosis compared to other anatomic sites. In an early study from 1970 by Balantyne et al from M.D. Anderson, the authors reported scalp subsite alone to be an independent predictor of poor survival.41 This trend continues to be identified in the literature today, with Leong et al reporting melanoma of the scalp to have the highest recurrence rate and a mortality that was three times greater than other subsites.21 In our study, we identified a sixfold increase in risk of distant recurrence in patients who had a negative SLNB with a scalp primary compared to those with a non‐scalp primary tumor.
Although we report a number of prognostic trends in CMHN, this study is not without limitations. First, our study is retrospective in nature over 22 years at a single institution. As such, some included patient cases were before institutional transition to electronic medical records and had variable length of detail in documentation with an inconsistent template. Additionally, records were identified using CPT codes that may have missed certain cases that may have met inclusion criteria. The TPR reported in various literature has significant variability from as low as 6.8% to as high as 27.8%.3, 8, 9, 15, 16, 17, 19, 23, 25 Our TPR was found to be 3.6%, which is lower than reported in the literature. Despite this, we feel the range of TPR in the literature is an inherent weakness of SLNB in HN melanoma. Additionally, some papers quoting TPR approaching 20% solely examined scalp melanomas, which are known to be more often positive and more aggressive.27 This study focused on identifying predictive preoperative and intraoperative characteristics that were more likely to lead to recurrence and did not specifically investigate adjuvant treatments. However, a supplementary analysis demonstrated that patients with a negative SLNB who received targeted therapy/immunotherapy and radiation had a greater risk of recurrence; this is likely due to more advanced disease as postoperative targeted therapy was significantly associated with higher Clark's level and T stage.
Our study generated additional outcomes representative of SLNB of the head and neck that further contribute to the entirety of literature on this subject. These results help better delineate predictors of recurrence and survival in head and neck melanoma and potentially improve patient outcomes through the statistical evaluation of recurrence following SLNB.
5. CONCLUSION
We report a number of prognostic trends in head and neck melanoma. SLN positivity was found more often in patients with a primary tumor of the scalp. Regional recurrence was significantly associated with age and tumor stage, whereas distant recurrence was significantly associated with tumor staging and scalp subsite. Scalp subsite was associated with an increased risk for nodal metastasis and distant recurrence. These findings support further investigation and rigorous surveillance of melanoma in this anatomic location.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Supporting information
Table S1 Number (percent) of patients who received radiation treatment or targeted therapy stratified by tumor stage
Table S2. Number (percent) of patients who received radiation treatment or targeted therapy stratified by Clarks level
Table S3. Univariable cox regression analysis of regional and distant recurrence in patients with negative SLNB (n = 148)a
ACKNOWLEDGMENTS
The research described was supported by NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI (Clinical and Translational Science Institute) Grant Numbers UL1TR001881 and UL1TR000124UCLA.
Echanique KA, Ghazizadeh S, Moon A, et al. Head & neck melanoma: A 22‐year experience of recurrence following sentinel lymph node biopsy. Laryngoscope Investigative Otolaryngology. 2021;6(4):738–746. 10.1002/lio2.605
Presented at: Academy of Otolaryngology Head & Neck Surgery Annual Meeting, New Orleans, LA. September 16, 2019.
Funding information NIH/National Center for Advancing Translational Science, Grant/Award Numbers: UL1TR000124UCLA, UL1TR001881
BIBLIOGRAPHY
- 1.Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127(4):392‐399. [DOI] [PubMed] [Google Scholar]
- 2.Saltman BE, Ganly I, Patel SG, et al. Prognostic implication of sentinel lymph node biopsy in cutaneous head and neck melanoma. Head Neck. 2010;32(12):1686‐1692. [DOI] [PubMed] [Google Scholar]
- 3.Erman AB, Collar RM, Griffith KA, et al. Sentinel lymph node biopsy is accurate and prognostic in head and neck melanoma: SLNB in head and neck melanoma. Cancer. 2012;118(4):1040‐1047. [DOI] [PubMed] [Google Scholar]
- 4.Main BG, Coyle MJ, Godden A, Godden DR. The metastatic potential of head and neck cutaneous malignant melanoma: is sentinel node biopsy useful? Br J Oral Maxillofac Surg. 2014;52(4):340‐343. [DOI] [PubMed] [Google Scholar]
- 5.Morton DL, Cochran AJ, Thompson JF, et al. Sentinel node biopsy for early‐stage melanoma: accuracy and morbidity in MSLT‐I, an international multicenter trial. Ann Surg. 2005;242(3):302‐311. discussion 311‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al‐Qurayshi Z, Hassan M, Srivastav S, et al. Risk and survival of patients with head and neck cutaneous melanoma: National perspective. Oncology. 2017;93(1):18‐28. [DOI] [PubMed] [Google Scholar]
- 7.Shah JP, Kraus DH, Dubner S, Sarkar S. Patterns of regional lymph node metastases from cutaneous melanomas of the head and neck. Am J Surg. 1991;162(4):320‐323. [DOI] [PubMed] [Google Scholar]
- 8.Hafström A, Romell A, Ingvar C, Wahlberg P, Greiff L. Sentinel lymph node biopsy staging for cutaneous malignant melanoma of the head and neck. Acta Otolaryngol. 2016;136(3):312‐318. [DOI] [PubMed] [Google Scholar]
- 9.Carlson GW, Page AJ, Cohen C, et al. Regional recurrence after negative sentinel lymph node biopsy for melanoma. Ann Surg. 2008;248(3):378‐386. [DOI] [PubMed] [Google Scholar]
- 10.Davis‐Malesevich MV, Goepfert R, Kubik M, Roberts DB, Myers JN, Kupferman ME. Recurrence of cutaneous melanoma of the head and neck after negative sentinel lymph node biopsy. Head Neck. 2015;37(8):1116‐1121. [DOI] [PubMed] [Google Scholar]
- 11.Ahmadzadehfar H, Hinz T, Wierzbicki A, et al. Sensitivity and false negative rate of sentinel lymph node biopsy (SLNB) in malignant melanoma of different parts of the body. J Nucl Med. 2014;55(suppl 1):1686‐1686. [Google Scholar]
- 12.Chao C, Wong SL, Edwards MJ, et al. Sentinel lymph node biopsy for head and neck melanomas. Ann Surg Oncol. 2003;10(1):21‐26. [DOI] [PubMed] [Google Scholar]
- 13.Hoetzenecker W, Guenova E, Böttinger TU, Häfner H‐M, Breuninger H. Mapping of specific sentinel node locations for skin cancer of the head. Eur J Dermatol. 2011;21(3):354‐358. [DOI] [PubMed] [Google Scholar]
- 14.Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence‐based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahimi‐Nedjat RK, Al‐Nawas B, Tuettenberg A, Sagheb K, Grabbe S, Walter C. Sentinel lymph node biopsy in malignant melanoma of the head and neck. J Cranio‐Maxillofac Surg. 2018;46(6):1027‐1031. [DOI] [PubMed] [Google Scholar]
- 16.Patuzzo R, Maurichi A, Camerini T, et al. Accuracy and prognostic value of sentinel lymph node biopsy in head and neck melanomas. J Surg Res. 2014;187(2):518‐524. [DOI] [PubMed] [Google Scholar]
- 17.Leiter U, Eigentler TK, Häfner H‐M, et al. Sentinel lymph node dissection in head and neck melanoma has prognostic impact on disease‐free and overall survival. Ann Surg Oncol. 2015;22(12):4073‐4080. [DOI] [PubMed] [Google Scholar]
- 18.Kachare SD, Brinkley J, Wong JH, Vohra NA, Zervos EE, Fitzgerald TL. The influence of sentinel lymph node biopsy on survival for intermediate‐thickness melanoma. Ann Surg Oncol. 2014;21(11):3377‐3385. [DOI] [PubMed] [Google Scholar]
- 19.Faut M, Wevers KP, van Ginkel RJ, et al. Nodular histologic subtype and ulceration are tumor factors associated with high risk of recurrence in sentinel node‐negative melanoma patients. Ann Surg Oncol. 2017;24(1):142‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parrett BM, Kashani‐Sabet M, Singer MI, et al. Long‐term prognosis and significance of the sentinel lymph node in head and neck melanoma. Otolaryngol Head Neck Surg. 2012;147(4):699‐706. [DOI] [PubMed] [Google Scholar]
- 21.Leong SPL, Accortt NA, Essner R, et al. Impact of sentinel node status and other risk factors on the clinical outcome of head and neck melanoma patients. Arch Otolaryngol Head Neck Surg. 2006;132(4):370‐373. [DOI] [PubMed] [Google Scholar]
- 22.Miller MW, Vetto JT, Monroe MM, Weerasinghe R, Andersen PE, Gross ND. False‐negative sentinel lymph node biopsy in head and neck melanoma. Otolaryngol Head Neck Surg. 2011;145(4):606‐611. [DOI] [PubMed] [Google Scholar]
- 23.de Rosa N, Lyman GH, Silbermins D, et al. Sentinel node biopsy for head and neck melanoma: a systematic review. Otolaryngol Head Neck Surg. 2011;145(3):375‐382. [DOI] [PubMed] [Google Scholar]
- 24.Gomez‐Rivera F, Santillan A, McMurphey AB, et al. Sentinel node biopsy in patients with cutaneous melanoma of the head and neck: recurrence and survival study. Head Neck. 2008;30(10):1284‐1294. [DOI] [PubMed] [Google Scholar]
- 25.Thomas DC, Han G, Leong SP, et al. Recurrence of melanoma after a negative sentinel node biopsy: predictors and impact of recurrence site on survival. Ann Surg Oncol. 2019;26(7):2254‐2262. [DOI] [PubMed] [Google Scholar]
- 26.Kulichová D, Dáňová J, Kunte C, Ruzicka T, Celko AM. Risk factors for malignant melanoma and preventive methods. Cutis. 2014;94(5):241‐248. [PubMed] [Google Scholar]
- 27.Cappello ZJ, Augenstein AC, Potts KL, McMasters KM, Bumpous JM. Sentinel lymph node status is the most important prognostic factor in patients with melanoma of the scalp. Laryngoscope. 2013;123(6):1411‐1415. [DOI] [PubMed] [Google Scholar]
- 28.Matthews NH, Li W‐Q, Qureshi AA, Weinstock MA, Cho E. Epidemiology of melanoma. In: Ward WH, Farma JM, eds. Cutaneous Melanoma: Etiology and Therapy. Brisbane (AU): Codon Publications; 2018. [PubMed] [Google Scholar]
- 29.Puza CJ, Josyula S, Terando AM, et al. Does the number of sentinel lymph nodes removed affect the false negative rate for head and neck melanoma? J Surg Oncol. 2018;117(7):1584‐1588. [DOI] [PubMed] [Google Scholar]
- 30.de Wilt JHW, Thompson JF, Uren RF, et al. Correlation between preoperative lymphoscintigraphy and metastatic nodal disease sites in 362 patients with cutaneous melanomas of the head and neck. Ann Surg. 2004;239(4):544‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmalbach CE, Nussenbaum B, Rees RS, Schwartz J, Johnson TM, Bradford CR. Reliability of sentinel lymph node mapping with biopsy for head and neck cutaneous melanoma. Arch Otolaryngol Head Neck Surg. 2003;129(1):61‐65. [DOI] [PubMed] [Google Scholar]
- 32.Morton DL, Wen DR, Foshag LJ, Essner R, Cochran A. Intraoperative lymphatic mapping and selective cervical lymphadenectomy for early‐stage melanomas of the head and neck. J Clin Oncol. 1993;11(9):1751‐1756. [DOI] [PubMed] [Google Scholar]
- 33.Bostick P, Essner R, Sarantou T, et al. Intraoperative lymphatic mapping for early‐stage melanoma of the head and neck. Am J Surg. 1997;174(5):536‐539. [DOI] [PubMed] [Google Scholar]
- 34.Jansen L, Koops HS, Nieweg OE, et al. Sentinel node biopsy for melanoma in the head and neck region. Head Neck. 2000;22(1):27‐33. [DOI] [PubMed] [Google Scholar]
- 35.Ollila DW, Foshag LJ, Essner R, Stern SL, Morton DL. Parotid region lymphatic mapping and sentinel lymphadenectomy for cutaneous melanoma. Ann Surg Oncol. 1999;6(2):150‐154. [DOI] [PubMed] [Google Scholar]
- 36.Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel‐node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370(7):599‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tas F, Erturk K. Scalp melanoma is associated with high mitotic rate and is a poor prognostic factor for recurrence and outcome. Melanoma Res. 2017;27(4):387‐390. [DOI] [PubMed] [Google Scholar]
- 38.Ozao‐Choy J, Nelson DW, Hiles J, et al. The prognostic importance of scalp location in primary head and neck melanoma. J Surg Oncol. 2017;116(3):337‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie C, Pan Y, McLean C, Mar V, Wolfe R, Kelly JW. Scalp melanoma: distinctive high risk clinical and histological features. Australas J Dermatol. 2017;58(3):181‐188. [DOI] [PubMed] [Google Scholar]
- 40.Jones EL, Jones TS, Pearlman NW, et al. Long‐term follow‐up and survival of patients following a recurrence of melanoma after a negative sentinel lymph node biopsy result. JAMA Surg. 2013;148(5):456‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ballantyne AJ. Malignant melanoma of the skin of the head and neck. An analysis of 405 cases. Am J Surg. 1970;120(4):425‐431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Number (percent) of patients who received radiation treatment or targeted therapy stratified by tumor stage
Table S2. Number (percent) of patients who received radiation treatment or targeted therapy stratified by Clarks level
Table S3. Univariable cox regression analysis of regional and distant recurrence in patients with negative SLNB (n = 148)a


