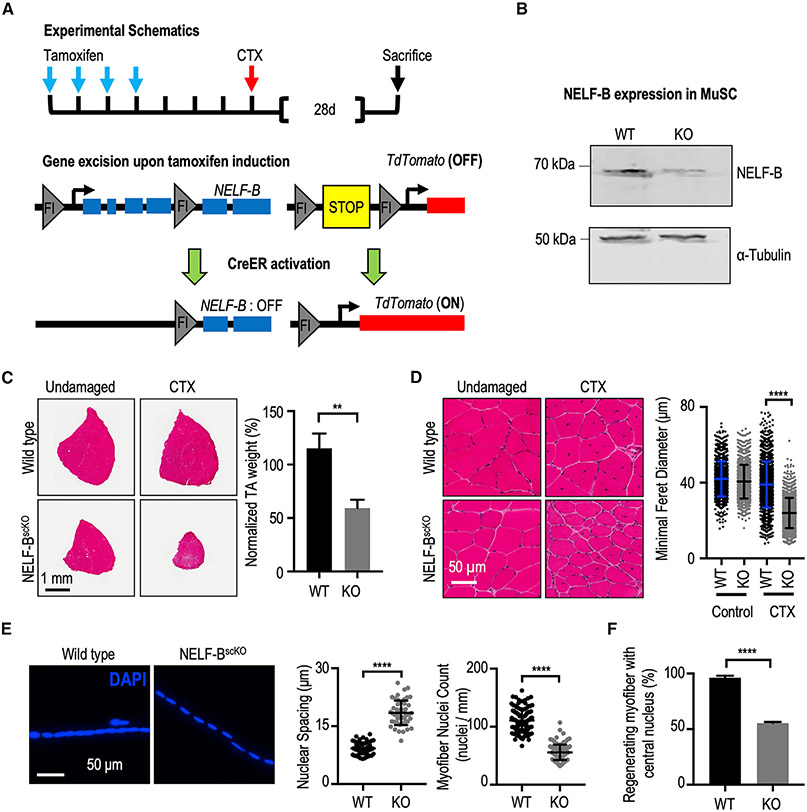
Figure 1. NELF is required for efficient MuSC-mediated myofiber repair after injury.
(A) Schematic representation of the recombination strategy. Tamoxifen administered in vivo activates CreERT2 to excise floxed genomic sequences. This is followed with intramuscular injection(s) of cardiotoxin to induce skeletal muscle damage.
(B) Western blot of a MuSCs whole cell extract isolated from WT (Pax7CreER/TdTscKO) and NELF-BscKO (NELF-BscKOPax7CreERTdTscKO) mice shows a 90% deletion efficiency of NELF-B.
(C) Hematoxylin and eosin stain of TA muscle cross-sections at 28 days post-injury (CTX), scale bar = 1 mm. The weight of the regenerated TA is presented relative to that of the undamaged contralateral leg and shows a reduction in size in the NELF-BscKO mice compared with WT controls (±SE, p-value < 0.01, n = 3).
(D) Magnified view of the regenerated and undamaged TA cross-sections shown in (C) demonstrate a reduced minimal Feret’s myofiber diameter in the regenerated NELF-BscKO myofibers (±SE, p-value < 0.0001, n = 3), scale bar = 50 μm.
(E) Single myofibers isolated from the regenerated EDL at 28 days post-injury were stained with DAPI to visualize nuclei from NELF-BscKO (n = 50 myofibers from 3 biological replicates) and WT (n = 57 myofibers from 3 biological replicates) mice, scale bar = 50 μm. Internuclear spacing is measured as the distance between the center of adjacent nuclei, whereas the myonuclei count was determined over the length of the myofiber (±SE, p-value < 0.0001).
(F) The relative abundance of newly regenerated myofibers in which the centrally located nucleus is captured in a 10 μm cross-section was calculated with tissues shown in (C) (±SE, p-value < 0.0001, n = 3).