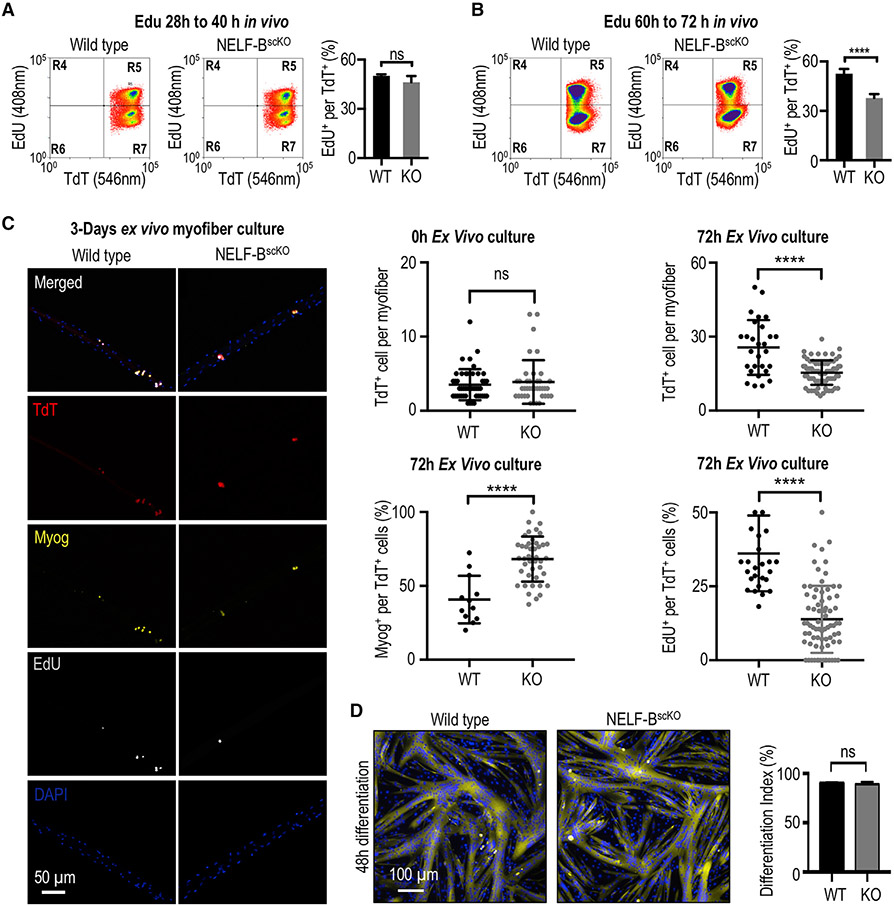
Figure 2. NELF is required for the massive expansion of muscle progenitors in response to injury.
(A) FACS-acquired quantification of EdU+ MuSCs (identified as the TdT+/ITGA7+ population) derived from the skeletal muscle 40 h post-injury (±SE, n = 4).
(B) FACS-acquired quantification of EdU+ MuSCs (identified as the TdT+/ITGA7+ population) derived from the skeletal muscle 72 h post-injury to monitor in vivo myoblast proliferation (±SE, p-value < 0.0001, n = 5).
(C) Single myofibers isolated from the EDL muscle from NELF-BscKO (n = 47 from 3 biological replicates) or WT (n = 46 from 3 biological replicates) mice were cultured (0 or 72 h) and stained with antibodies as indicated, scale bar = 50 μm. Quantification of TdT+, EdU+, and Myog+ cells are shown (±SE, *p-value < 0.0001).
(D) Primary myoblasts isolated from NELF-BscKO and WT controls were plated at high density and induced to undergo terminal differentiation, scale bar = 100 μm. The differentiation index was calculated as the percentage of nuclei present in multinucleated myotubes (±SE, n = 3).