Fig. 2. Descriptive summary of the 89 clinical trials using cell therapies for COVID-19.
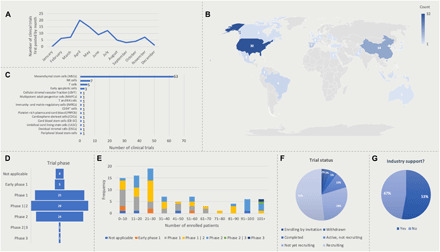
(A) Number of COVID-19 targeting cell therapy clinical trials started in each month of the year 2020. (B) World map showing global distribution of the registered cell therapy clinical trials and their numbers per country. (C) Different cell types used in the cell therapy–based clinical trials and their respective count. (D) Stages of the 89 cell therapy clinical trials registered as of 1 January 2021. (E) Distribution of patient enrollment numbers across the 89 clinical trials. (F) Breakdown of the 89 cell therapy clinical trial statuses. (G) The percentages of cell therapies sponsored and supported by the industry sector.
