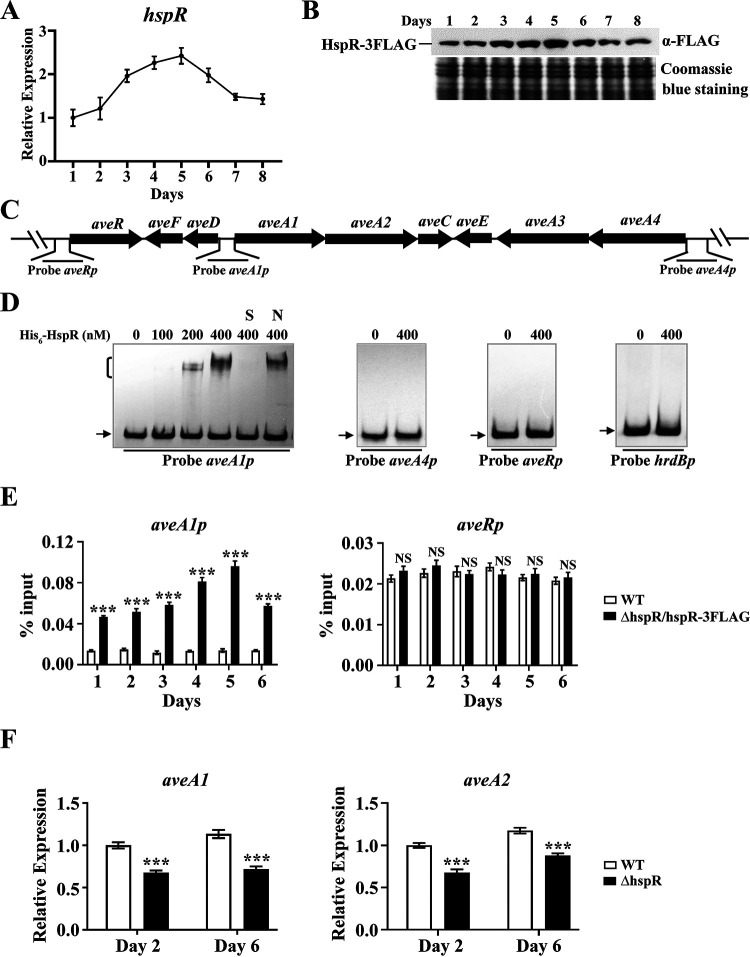
FIG 2.
Direct activation of aveA1-aveA2 by HspR. (A) RT-qPCR analysis of hspR transcriptional pattern in the WT grown in FM-I. The hspR transcription level on day 1 was set to 1. (B) Western blotting analysis of the HspR protein level during the fermentation process. The HspR expression level in strain ΔhspR/hspR-3FLAG grown in FM-I was determined using anti-FLAG MAb. (C) Schematic diagram of promoter probes used in EMSAs. (D) In vitro EMSAs of interactions of His6-HspR with indicated probes (hrdBp as negative-control probe). A 0.15 nM concentration of labeled probe and various amounts of His6-HspR were used for each binding reaction. In competition experiments, ∼300-fold unlabeled nonspecific probe hrdBp (lane N) or specific probe aveA1p (lane S) was used. Arrows indicate free probes. Bracket indicates the HspR-DNA complex. (E) In vivo ChIP-qPCR assays of HspR binding to aveA1p and aveRp using ΔhspR/hspR-3FLAG and WT (negative control) strains grown in FM-II for the indicated times. The y axis indicates the relative binding level of HspR-FLAG on each site, determined by recovery of target sequence with anti-FLAG MAb. (F) RT-qPCR analysis of aveA1 and aveA2 in WT and ΔhspR strains grown in FM-I. WT value on day 2 for each gene was set to 1. Error bars (panels A, E, and F) indicate SDs from three replicates. NS, not significant; ***, P < 0.001 (t test).