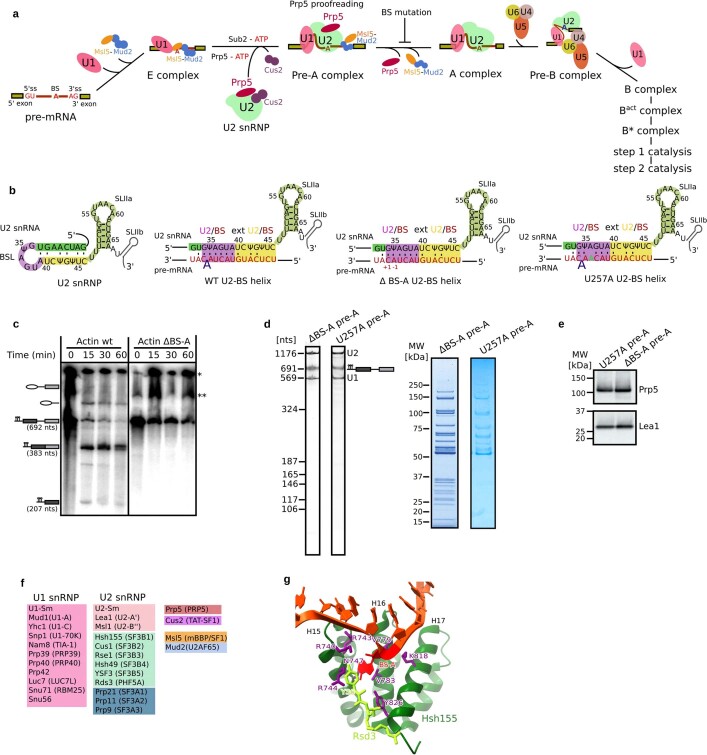
Extended Data Fig. 1. Biochemical characterization of S. cerevisiae pre-A spliceosomal complexes.
a, Early assembly of the S. cerevisiae spliceosome. Whereas Prp5 and Tat-SF1 (Cus2 in yeast) are stable components of the human 17S U2 snRNP, they appear to be less-stably associated with the yeast U2 snRNP. The spliceosome undergoes numerous structural and compositional rearrangements during its assembly and catalysis of pre-mRNA splicing34,35. Conserved DEXH/D-box RNA helicases are important driving forces for these rearrangements, and also ensure the proper recognition of the branch site (BS) and the 5′- and 3′-splice sites (ss) via proofreading mechanisms36,37. Initially an E complex is formed in an ATP-independent manner. In the yeast E complex (also denoted the commitment complex), the 5′-ss is bound by U1 snRNP, and the BS and 3′-end of the intron are bound by a heterodimer of Msl5 and Mud2. RNP rearrangements that lead to the stable association of U2 snRNP and enable the formation of a U2–BS helix—in which an adenosine is bulged, specifying it as the nucleophile for catalytic step 1 of splicing—require the ATP-dependent action of the DEAD-box RNA helicases Sub2 (refs. 38,39; UAP56 in humans) and Prp5 (refs. 1–4,40). U2 nucleotides that base pair with the BS are initially sequestered in a stem-loop structure denoted the BSL11,14. Sub2 may free the BS region by displacing Msl5 (refs. 41,42), while Prp5 has been proposed to displace U2 snRNP proteins, including Cus2 (TAT–SF1 in humans), from the BSL11,14. This frees U2 nucleotides to base pair with the BS, and leads to the formation of the A complex with stably bound U2 snRNP. b, Structure of the BSL and U2–BS helices formed on an Act pre-mRNA wild-type (WT) BS (UACUA(A)C, where the BS-A is in bold), ΔBS-A (UACUAC) or U257A (UACAA(A)C) branch site. Note that the exact conformation of the U257A U2–BS helix is not clear. The U2–BS helix is highlighted in purple, and the extended U2–BS helix, in which the number and nature of base-pairing interactions varies depending on the pre-mRNA intron sequence, is highlighted in yellow. c, Deletion of the BS adenosine from the Act pre-mRNA stalls splicing before the first catalytic step. Splicing was performed in two independent experiments in yeast extract for 30 min at 23 °C with wild-type (lane 1) or ΔBS-A (lane 2) Act pre-mRNA containing MS2 aptamers for affinity purification. *Position of the loading well. **Band artefact not related to pre-mRNA splicing. For gel source data, see Supplementary Fig. 1. d, RNA (left gels) and protein (right gels) composition of purified yeast pre-A complexes formed on ΔBS-A and U257A Act pre-mRNA. RNA and protein were analysed on NuPAGE gels and visualized by staining with SYBR Gold or Coomassie, respectively, in two independent experiments. Note that fewer picomoles of the U257A pre-A complex were loaded onto the gel and, as a consequence, proteins of lower molecular weight are poorly or not at all visible. e, Prp5 is present in both U257A and ΔBS-A pre-A complexes. Proteins from affinity-purified U257A or ΔBS-A pre-A complexes (as indicated above each lane) were analysed by western blotting in two independent experiments with antibodies against S. cerevisiae Prp5 or Lea1 (used to ensure equal loading). f, Proteins localized in the S. cerevisiae pre-A complex and their human homologues (shown in parentheses). Only U1–70K, U1–A and U1–C have been identified as stable components of human U1 snRNPs. Human homologues of Snu56 and Prp42 have not been identified. g, Residues forming the BS-A-binding pocket. The bulged BS-A is bound in a pocket composed of residues R744, N747, V783 and Y826 of Hsh155 and residue Y36 of Rds3 (refs. 7,8,43). The BS-A ribose and 5′-phosphate are also located near Hsh155 residues K740 and K818, respectively. Most of these residues are evolutionarily highly conserved. A Hsh155 K818A mutation is lethal, as are mutations in residues of Hsh155 that contact the backbone of nucleotides directly adjacent to the bulged BS-A19. However, many of the Hsh155 residues forming the BS-A binding pocket are nonessential. That is, single alanine substitutions at K740, R744, N747 and V783 do not affect yeast viability, but they do affect recognition of the branch site19. Substitutions with bulkier amino acids decrease the use of nonconsensus branch sites, whereas substitutions with smaller amino acids increase usage19.