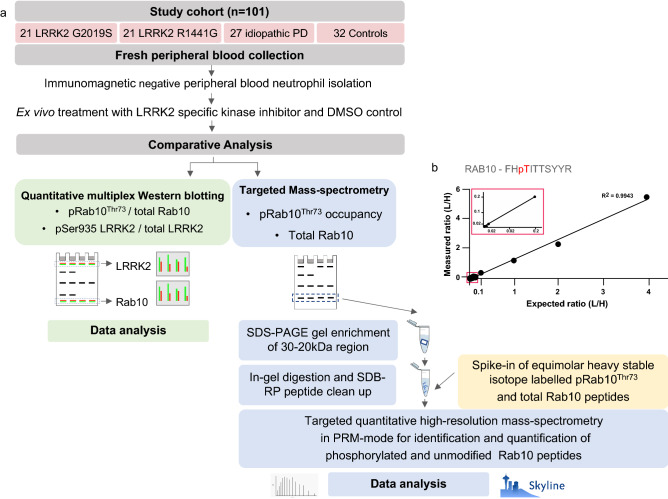
Fig. 1.
Experimental design and workflow for peripheral blood neutrophils.a 20 ml of fresh peripheral blood was collected from 101 participants with and without either PD and with or without a pathogenic LRRK2 mutation—LRRK2 G2019S or LRRK2 R1441G—for peripheral blood neutrophil isolation by immunomagnetic negative isolation. Purified neutrophils were then split into two parts for ex vivo treatment with either the specific LRRK2 kinase inhibitor MLi-2 (200 nM) or DMSO for 30 min prior to cell lysis and storage at -80 degrees Celsius. All sets of neutrophil lysates (MLi-2 and DMSO treated) were then subjected to quantitative multiplexed immunoblotting for pRab10Thr73 phosphorylation/total Rab10 protein ratio as well as targeted mass-spectrometry for pRab10Thr73 phosphorylation occupancy with spike in of heavy-labelled Rab10-phospho- and total peptide standards after enrichment by SDS–PAGE followed by in-gel digestion and subsequent mass-spectrometry and data analysis. Additionally, quantitative immunoblot analysis was performed for Serine 935 phosphorylation/ total LRRK2 protein as well as total Rab10 and total LRRK2 protein levels, both normalised against GAPDH. Limit of detection of targeted MS assay (b). Scatter plot depicting the limit of detection and quantification of pRab10Thr73 in targeted PRM. 50 fmol of heavy pRab10Thr73 was mixed with a variable amount of light pRab10 ranging from 0.01, 0.1, 1, 10, 50, 100 and 200 fmol that was spiked into 50 ng of HeLa lyastes. Light/heavy ratio values were plotted to show the linear response of pRab10Thr73 [0.01 to 200 fmol (R2 = 0.9943)). The zoom in rectangular box depicting the values of 0.01 to 0.1 fmol. n = 3, error bars representing mean and SD