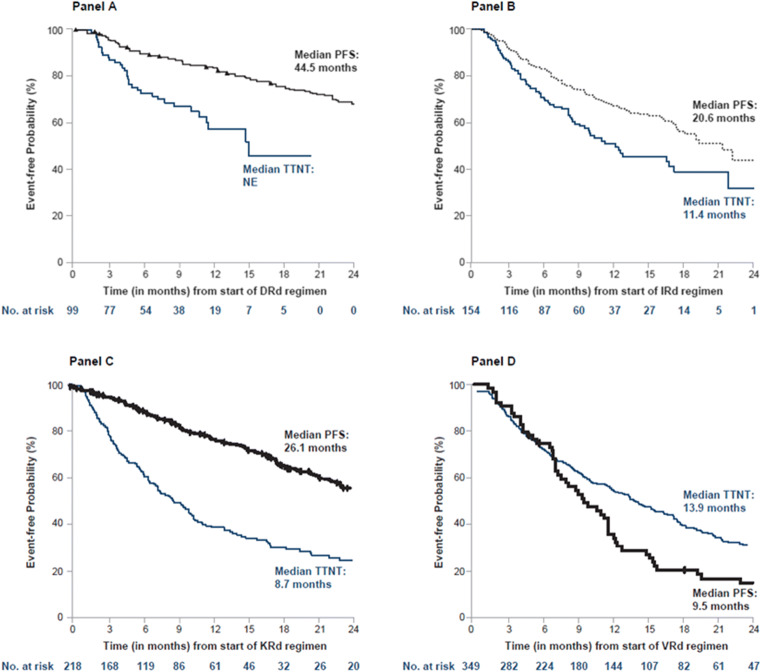
Fig. 2.
Real-world unadjusted TTNT vs PFS from hallmark trials in LOT ≥ 2 for DRd (a), IRd (b), KRd (c), and VRd (d). aAn event in the embedded clinical trials was defined as progression or death; an event in the real-world population in this study was defined as the start of the next line of therapy or death (as a proxy for PFS). bEmbedded PFS KM curves were adapted from a Bahlis NJ, et al. Leukemia. 2020 Jan 30. doi: 10.1038/s41375-020-0711-6; b Moreau P, et al. N Engl J Med. 2016;374:1621-1634; c Siegel DS, et al. J Clin Oncol. 2018 Mar 10;36(8):728-734; d Richardson PG, et al. Blood. 2014 Mar 6;123(10):1461-9. Key: DRd, daratumumab, lenalidomide, dexamethasone; IRd, ixazomib, lenalidomide, dexamethasone; KM, Kaplan-Meier; KRd, carfilzomib, lenalidomide, dexamethasone; LOT, line of therapy; PFS, progression-free survival; Rd, lenalidomide, dexamethasone; TTNT, time to next therapy; VRd, bortezomib, lenalidomide, dexamethasone