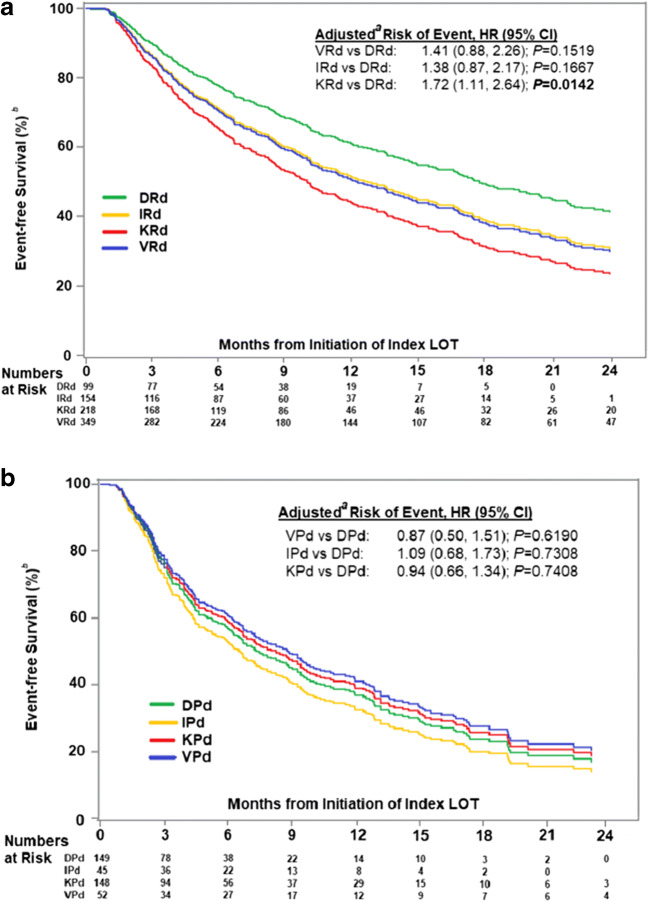
Fig. 3.
Real-world adjusted TTNT for Rd (a) and for Pd backbone triplets in LOT ≥ 2 (b), by regimen type. aAll survival analyses were stratified by LOT (2, 3, ≥ 4). Adjusted for age (18–64, 65–74, ≥ 75 years); CCI (0, 1, ≥ 2); EGOG PS (0–1, 2–4, unknown), presence of CRAB symptoms, presence of peripheral neuropathy, and prior SCT; cytogenetic risk (standard risk/unknown, high risk); ISS stage (I/II, III, unknown); prior treatment exposure (PI, IMID, PI+IMID, and none), prior daratumumab exposure; PI- and/or IMID-refractory status (PI-refractory, IMID-refractory, PI + IMID-refractory, and not refractory); time from diagnosis to start of index line of therapy (months); refractory to prior line of therapy (TFI of ≤ 60 days vs > 60 days); time from start of LOT1 to start of LOT2 (months); year of diagnosis (2007–2011, 2012–2015, 2016–2017); and setting of care (community, academic, unknown). bAn event was defined as the start of next line of therapy or death. Key: CRAB, hypercalcemia, renal insufficiency, anemia, bone disease; DRd, daratumumab, lenalidomide, dexamethasone; IMID, immunomodulator; IRd, ixazomib, lenalidomide, dexamethasone; ISS, International Staging System; KM, Kaplan-Meier; KRd, carfilzomib, lenalidomide, dexamethasone; LOT, line of therapy; PI, proteasome inhibitor; PFS, progression-free survival; Rd, lenalidomide, dexamethasone; SCT, stem cell transplant; TFI, treatment-free interval; TTNT, time to next therapy; VRd, bortezomib, lenalidomide, dexamethasone