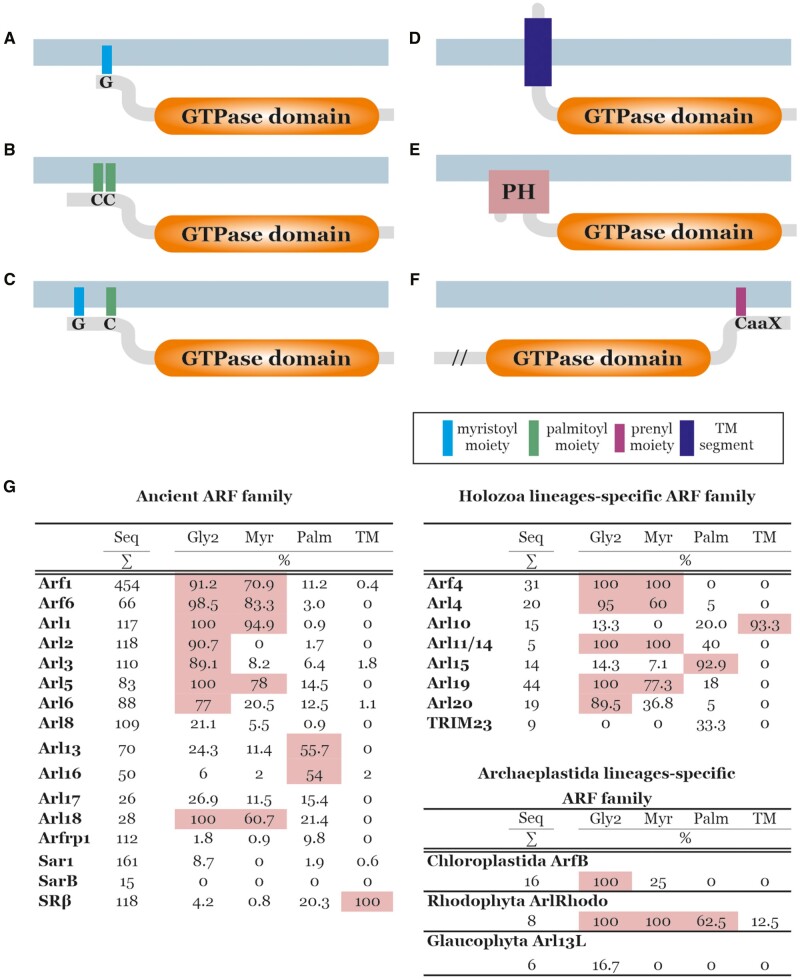
Fig. 7.
Membrane attachment mechanisms of ARF family proteins. Examples of different broadly conserved mechanisms of membrane attachments of ARF family members are depicted. (A) N-terminally myristoylated glycine residues, common for Arfs and several Arf-like proteins. (B) One or two S-palmitoylated cysteine residues near the N-terminus, typical for Arl16 and also common in Arl13. (C) N-terminally myristoylated glycine residue coupled with S-palmitoylated cysteine residue near the N-terminus, typical for ArlRhodo. (D) N-terminal transmembrane region, typical for SRβ and Arl10. (E) N-terminally accreted PH domain, present in divergent Arf-like proteins in kinetoplastids and choanoflagellates. (F) Prenylation motif (CaaX) at the C-terminus of certain eustigmatophyte-specific ARF family members (characterized also by a long N-terminal extension, in the figure marked with “//”). Supplementary table 1, Supplementary Material online, lists all identified ARF family proteins predicted to be N-myristoylated or S-palmitoylated, or to contain a transmembrane region or PH domain. (G) Summary of the results of prediction of N-myristoylation, S-palmitoylation, and presence of the transmembrane (TM) region in particular subgroups of the ARF family. For each subgroup (group of orthologs), the number of sequences (Seq) and the percentages of sequences with glycine residues at the second position (Gly2), sequences predicted as N-myristoylated (Myr), sequences predicted as S-palmitoylated on at least one cysteine residue (Palm), and sequences with predicted transmembrane region(s) (TM) are given. Values above 50% are highlighted in pink. For complete data, see supplementary tables 1 and 5, Supplementary Material online. These predictions were done as described under Materials and Methods.