Abstract
Background.
Annona muricata and Khaya grandifoliola are ethnomedicinally used for the treatment of malaria and have been experimentally shown to have an anti-plasmodial effect, but the mechanisms involved are not fully understood. This study investigated the effect of the ethanol extracts of their leaves on parasitemia, radical scavenging and cytokines in Plasmodium berghei ANKA-infected BALB/c mice.
Methods.
BALB/c mice were infected with P. berghei and treated with chloroquine, A. muricata or K. grandifoliola extract for 4 days. The percentage of parasitemia and the level of cytokine expression were determined after treatment. Trace element, phytochemical and nitric oxide (NO) scavenging activity, 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging properties assays were done to study the antioxidant effects of AN and KG in vitro.
Results.
P. berghei consistently increased parasitemia in BALB/c mice. The tested doses (100-, 200-, and 400 mg/kg) of A. muricata and K. grandifoliola attenuated the P. berghei-induced elevation of parasitemia and cytokines (TNF-α, IL-5, and IL-6) in vivo during the experimental period, though not as much as chloroquine. Moreover, both extracts scavenged the DPPH and NO radicals, though A. muricata had more anti-oxidant effect than K. grandifoliola in-vitro.
Conclusion.
The ethanol extracts of A. muricata and K. grandifoliola reduce parasitemia in P. berghei-treated mice BALB/c by scavenging free radicals and reducing cytokines, though the extracts were not as effective as chloroquine.
Keywords: Annona muricata, Khaya grandifoliola, Plasmodium berghei, BALB/c mice, cytokine inhibition, cerebral malaria
Introduction
Malaria is caused in humans by the six (6) Plasmodium species (among over 100 species) including P. falciparum, P. vivax, P. ovale, P. malariae, P. simiu and P. knowlesi.1 P. falciparum and P. vivax are the 2 species that mostly cause infection with severe anemia, though the most severe form of the disease (cerebral malaria, CM) is caused by P. falciparum. 2 Malaria is a tropical disease with a serious public health burden worldwide and a significant impact on Sub-Saharan Africa, as it is the major cause of mortality in middle-, low-income and developing countries. Globally, the WHO reported 200 million new cases of Plasmodium infections and 435,000 deaths in 2018.3 About 106 countries are considered endemic to malaria infection, with a 3.3 billion population at risk.4 In Uganda, 95% of the population is at risk of malaria infection,5 which kills 70,000 and 100,000 children and pregnant women respectively each year.6 The highest proportion of the malaria burden in Uganda affects children and pregnant women living in resource-limited settings such as hard-to-reach rural settlements, and poor people with limited access to health care services coupled with a lack of education.7
The CM is an encephalopathy with severe neurological impediment,8 and often, less than 20% of cases survive with neurological disabilities9 including cognitive and speech deficits, motor alterations and cortical blindness.10 There are several pathophysiological processes involved in CM syndrome. For instance, investigations have shown that disproportionate signals in the pro- and anti-inflammatory immune response, a disruption of the blood-brain barrier and an activation of the endothelial cell system are fundamental processes in the pathophysiological development of CM.11 Specifically, the overproductions of pro-inflammatory mediators such as tumor necrosis factor-alpha (TNF-α), gamma interferon (IFN-γ), interleukins, macrophage colony-stimulating factor (M-CSF), and lymphotoxin12 are pieces of evidence of the pathogenesis of CM.
Bioactive components from plant derivatives have been a pivotal source for the development of antimalarial therapeutics. For examples, chloroquine/quinine (Cinchona) 13 and artemisinin (herbal tree Artemisia annua) 14 are derived from plant constituents. Since the resistance against chloroquine was reported in 1957 in Thailand15 and in 1976 in India,16 malaria-endemic and the development of resistance to the current treatment spread globally, which has made malaria to be of great public health concern. A resistant strain of artemisinin and its derivatives,17 which are presently the first line of antimalarial drugs, was reported in the Thai-Cambodia region in 2009.18 Therefore, the need to search for new active antimalarial agents is important.
Evidence-based academic reports recognize Annona muricata Linn ethnomedicinal application in tropical regions for the management and treatment of diverse ailments, including fever,19 pain, respiratory and skin illness, internal and external parasitic infections, bacterial infections,19 inflammation,20 diabetes21 and cancer.22 Apart from the local use of A. muricata in Uganda,23 Togo, Cameroon and Vietnam24 to treat malaria, studies have reported the antimalarial activity of A. muricata in P. berghei ANKA25 and P. falciparum 26 infection models. Similarly, Khaya grandifoliola C.DC. has shown significant pharmacological potential as a hepatoprotective, antiviral and anti-inflammatory agent.27 Apart from the folk medicinal use of K. grandifoliola in Uganda28 and Nigeria to treat malaria and its symptoms like fever, its anti-malarial effect has been demonstrated against P. berghei and P. falciparum models of malaria.29 However, the mechanisms of their antimalarial effect need to be investigated.
Even though they can’t perfectly mimic the full human syndrome of the disease, malaria models have been developed in mice, monkey, and rats and they faithfully represent certain aspects of human malaria. The similarities between the human and murine antigens and immune response pathway have made the mouse a model of choice in malaria studies. Moreover, P. berghei is the most widely used parasite model of severe malaria infection in rodents because it sequesters within the microcirculation, which is the major characteristic of severe cerebral malaria.30 In this study, the antimalarial effect and the mechanism of action of the A. muricata and K. grandifoliola leaf extracts in P. berghei-infected BALB/c mice were investigated.
Methods
Plant Collection Authentication and Extractions
A. muricata and K. grandifoliola were harvested from the Rubirizi district, Southwest of Uganda. The specimen was authenticated by a taxonomist, and the identification number (HOPE-PHA-2019/01, 2019/02) was left in the School of Pharmacy herbarium, Kampala International University, Uganda, for future citation/references.
Fresh plant leaves were crammed into sterile polythene bags and transported to the Department of Pharmacology Laboratory. The samples were washed and air-dried at room temperature for approximately 1 month, crushed into powder using a clean mortar and pestle, and stored in the desiccator until ready for use. A. muricata and K. grandifoliola ethanolic extracts were obtained by applying the method described by.31 Briefly, 150 g of the plant powder was macerated on a separate jar in 900 ml of absolute ethanol and agitated every 24 hrs for 3 days. The solvent was decanted and filtered with Whatman No. 1 filter paper, followed by evaporation in a water bath at a temperature of 400°C and kept in the refrigerator until ready to use.
Trace Element Analysis
An atomic absorption spectrophotometer (AAS 969 Unicam Solar 32) was used to analyze the essential mineral content of the A. muricata and K. grandifoliola ethanolic extracts following the protocols of AOAC 2000, while Na and K were evaluated by flame photometry (JENWAY PF7).
Phytochemical Analysis
The presence of phytochemicals such as alkaloids, tannins, saponins, flavonoids, steroids, cardioglycosides, anthraquinones and triterpenoids was determined by standard methods reported by Trease and Evans.32
Induction of Parasitemia and Experimental Treatment Regimens
The chloroquine-sensitive P. berghei strain was sourced from the Uganda Medical Research Institute, Kampala. Mice were infected intraperitoneally (i.p.) with 0.2 107 ml parasitized red blood cells (RBCs). Before the commencement of the study, the number of parasitemia was observed and counted with a hemocytometer, while the parasites were adjusted to 0.5 × 106 in phosphate-buffered saline (PBS) sterile solution. Aside from the 4 mice that were used for the adjustment of parasitemia and another 15 mice used for the toxicity study, a total of 54 mice were divided into 9 groups (n = 6 per group) following the method of Charan and Kantharia33 for the determination of animal sample size. The treatment groups were intraperitoneally injected with 200 µl inoculum of 0.5×106 parasites (named Day 0), and treatment started on day 1 up to day 4.
Group 1—Control group: parasitemia (day 0, i.p.) + distill water (placebo)
Group 2—Treatment group: parasitemia (day 0, i.p.) + A. muticata (100 mg/kg, p.o., day 1-4)
Group 3—Treatment group: parasitemia (day 0, i.p.) + A. muticata (200 mg/kg, p.o., day 1-4)
Group 4—Treatment group: parasitemia (day 0, i.p.) + A. muticata (400 mg/kg, p.o., day 1-4)
Group 5—Treatment group: parasitemia (day 0, i.p.) + K. grandifoliola (100 mg/kg, p.o., day 1-4)
Group 6—Treatment group: parasitemia (day 0, i.p.) + K. grandifoliola (200 mg/kg, p.o., day 1-4)
Group 7—Treatment group: parasitemia (day 0, i.p.) + K. grandifoliola (400 mg/kg, p.o., day 1-4)
Group 8—Drug control group: parasitemia (day 0, i.p.) + Chloroquine (5mg/kg, p.o., day 1-4)
Group 9—Normal group: distilled water (day 0-4).
Before treatments, indicator and physical signs of illness30 such as piloerection, lethargy, reduction in locomotor, and passage of dark urine were checked in the parasite-infected mice and control on days 1 and 2. It was observed to be moderate in the groups on day 2, which became severe in the untreated mice on day 4 and mild in the other groups.
Antimalarial Activity of the Extracts
Thin blood smears were prepared from tail blood for days 0-4 for the percentage parasitemia level and suppressive test. The smear was fixed with methanol, allowed to dry for 15 min and stained with 10% Giemsa stain at pH 7.2 for 15 min and acridine orange. Afterward, the stained slides were washed and allowed to dry at room temperature. The slides for each mouse were examined under a UV illumination microscope (Olympus) with an oil immersion adjusted objective of 100× magnification. Each slide in different fields was examined, and the number of parasitemia counts was expressed as shown below.
% Parasitemia = (Number of parasitized RBCs / Total number of RBCs counted) × 100
% suppression = mean parasitemia of untreated group / Mean parasitemia of treated group X 100
Antioxidant Activity of the Extracts
DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical scavenging activity of the ethanolic extracts
The scavenging ability of the extracts for free radicals was measured by assaying the DPPH radical scavenging activity as reported by Braca et al34 An aliquot of 2 ml of 0.04 % DPPH solution in ethanol was mixed with 1.0 ml of each ethanolic extract/ascorbic acid of different concentrations (20, 40, 60, 80, 100 mg/ml) and vortexed and then allowed to attain a steady-state for 30 minutes at room temperature in a dark chamber. The inhibitory activity of DPPH by the extracts was determined by measuring the absorbance at 517 nm against a control (1 ml ethanol plus 2 ml of DPPH). The percentage inhibition of DPPH radical scavenging ability of the extract was expressed as follows:
% Inhibition = [(Ac-As)/ Ac] * 100, where Ac is absorbance of control; As is defined as the absorbance of extracts/ascorbic acid.
Nitric oxide scavenging activity of the ethanolic extracts
The nitric oxide (NO) scavenging activity of the ethanolic extracts was measured following the protocol of Braca et al, (2001).34 A 0.5 ml aliquot of the different concentrations (20, 40, 60, 80 and 100 μ/ml) was mixed with 2 ml of 10 mM sodium nitroprusside dissolved in 0.5 ml phosphate buffer saline (pH 7.4). The assortment was incubated at room temperature for 2 hours and 30 minutes. Afterward, 1 ml of incubated solution was added to 1 ml of Nedd reagent and incubated at room temperature for 30 minutes. Absorbance was measured using a spectrophotometer at 540 nm. Ascorbic acid was used as a standard. Blank was 1 ml of water, 2 ml of sodium nitroprusside and 1 ml of Nedd reagent. The control was 2 ml of sodium nitroprusside, 0.5 ml of phosphate buffer, 1 ml of Nedd reagent and 0.5 ml of methanol. The number of nitric oxide radicals scavenged was determined using the formula shown below:
% Inhibition = [(Ac-As)/ Ac] * 100
where Ac = Absorbance of the control and As = Absorbance of the plant extract.
Acute Toxicity Test
The standard protocol of CDER (1996) and WHO (1992) to investigate the acute toxicity of the extracts was adopted as described before35 while the LD50 values for the 2 extracts were calculated according to Miller and Tainter.36 A total of 25 mice was used for the toxicity study.
Cytokine Assays
Blood was collected from all groups by inserting a needle into the vein starting at the tip of the tail37,38 days 1, 3 and 6. The blood was centrifuged at 1,000 g for 10 min to obtain serum. Cytokine ELISA kits (R&D System, USA) for tumor necrosis factor-alpha (TNF-α) and interleukin (IL-5 and IL-6) were assayed according to the manufacturer’s instructions.
Statistical Analysis
Data were blindly analyzed with SPSS version 16.0 and expressed as the mean ± standard error of the mean. The percentage inhibition of DPPH and NO and the levels of cytokines were compared by 2-way analysis of variance (ANOVA), followed by the posthoc Bonferroni test for multiple comparisons. To control for the differences in the baseline value (which is a co-variate) of the parasitemic rats, analysis of covariance (ANCOVA) was used to compare the parasitemia in all the groups, followed by a posthoc Bonferroni test. P < 0.005 was considered statistically significant.
Results
Phytochemical and Trace Elements in the AM and K. grandifoliola Extracts
Both A. muricata and K. grandifoliola contain flavonoids, saponnins, tannins, alkaloids, triterpenoids, reducing sugars and cardiac glycosides but not steroids (Table 1). Moreover, the A. muricata and K. grandifoliola had 9 and 7 essential elements respectively, with sodium (39 mg/100 g and 30.443 mg/100 g) and potassium (56.121 mg/100 g and 15.621 mg/100 g) respectively dominating both extracts (Table 2).
Table 1.
Phytochemical Screening of the Ethanol Extracts of A. muricata and K. grandifoliola.
| Phytochemicals | A. muricata and | K. grandifoliola |
|---|---|---|
| Flavonoid | + | + |
| Saponins | + | + |
| Tannins | + | + |
| Steroids | − | − |
| Alkaloids | + | + |
| Anthraquinones | − | + |
| Triterpenoids | + | + |
| Reducing Sugar | + | + |
| Cardiac glycoside | + | + |
+, Present; −, Absent.
Table 2.
Trace Elements in the Ethanol Extracts of A. muricata and K. grandifoliola.
| Elements (ppm) | A. muricata | K. grandifoliola |
|---|---|---|
| Sodium | 39 | 30.443 |
| Potassium | 56.121 | 15.621 |
| Magnesium | 34.508 | 10.411 |
| Iron | 5.008 | 3.067 |
| Lead | 0.332 | 0 |
| Copper | 0.424 | 1.016 |
| Manganese | 0.201 | 0.121 |
| Zinc | 2.899 | 0.100 |
| Nickel | 0.022 | 0 |
Acute Toxicity Test
There were no behavioral changes observed after the administration of the different doses of both extracts, and no death was recorded up to 7 days of the observation period. There was also a reduced aggressiveness and movement among the extract-treated groups compared to the control group during the first 4 hours. These indicate that the extracts are relatively safe at 2500 mg/kg with a greater LD50. Therefore, the extracts are considered safe at the tested doses and practically nontoxic using Cotonat J (1996)39 classification of a range of LD50.
A. muricata and K. grandifoliola Reduced Parasitemia in P. berghei-Infected BALB/c Mice
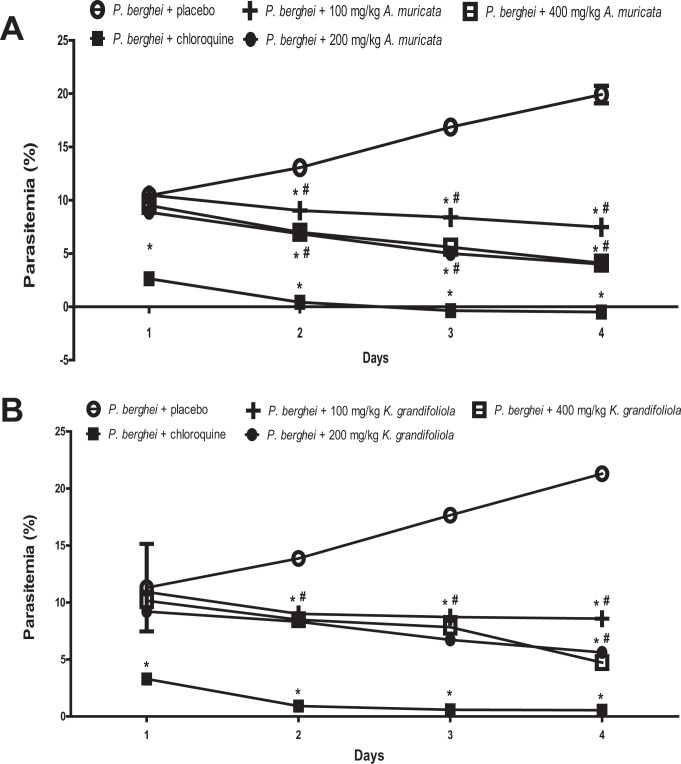
Parasitemia was increased in P. berghei-infected BALB/c mice treated with placebo but reduced in P. berghei-infected BALB/c mice treated with A. muricata, K. grandifoliola, and chloroquine. In order to determine the impacts of A. muricata and K. grandifoliola on the daily parasitemia level, the adjusted means in rats that received A. muricata or K. grandifoliola was compared to those that received placebo and chloroquine. It was observed that none of the 3 doses of A. muricata and K. grandifoliola reduced parasitemia as much as chloroquine (Figure 1).
Figure 1.
A. muricata (A) and K. grandifoliola (B) extracts reduced parasitemia in P. berghei-infected BALB/c mice. *P < 0.05 vs. P. berghei + placebo of the same day; # p < 0.05 vs. P. berghei + chloroquine of the same day.
On day 1, only 200 mg/kg but not 100 mg/kg and 400 mg/kg of A. muricata significantly reduced the parasitemia in P. berghei-infected BALB/c mice when compared to the infected mice treated with placebo. Furthermore, all doses of A. muricata significantly reduced the parasitemia at days 2-4 when compared to the corresponding level in the infected mice treated with placebo (Figure 1A).
Similarly, on day 1, only 200 mg/kg but not 100 mg/kg and 400 mg/kg of K. grandifoliola significantly reduced the parasitemia in P. berghei-infected BALB/c mice when compared to the infected mice treated with placebo. Also, all doses of K. grandifoliola significantly reduced the parasitemia at days 2-4 when compared to the corresponding level in the infected mice treated with placebo (Figure 1B).
The percentage suppression of parasitemia by the extracts showed the same pattern as the parasitemia data, and is therefore not shown in the figures.
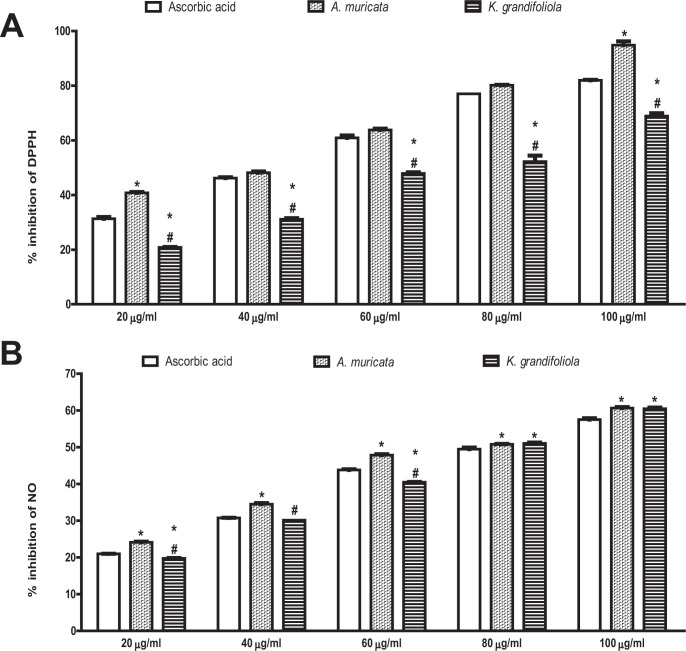
In-Vitro Antioxidant Activity of the Ethanolic Extracts of A. muricata and K. grandifoliola
The A. muricata caused higher percentage inhibition of DPPH radical at 20 µg/ml and 100 µg/ml but the same percentage inhibition of DPPH radical as ascorbic acid at 40 µg/ml, 60 µg/ml and 80 µg/ml. On the contrary, all the doses of K. grandifoliola caused lower percentage inhibition of DPPH radical than ascorbic acid. Comparatively, K. grandifoliola caused lower percentage inhibition of DPPH radical than A. muricata across all the doses, even though the percentage inhibition of both extracts increased in a dose-dependent manner (Figure 2A).
Figure 2.
A. muricata and K. grandifoliola extracts scavenge DPPH (A) & NO (B) radicals in vitro. Each bar represents the mean ± S.E.M of triplicate experiments. DPPH, 2,2-diphenyl-1-picrylhydrazyl; NO, Nitric oxide; *p < 0.05 vs. ascorbic acid of the same dose; # p < 0.05 vs. A. muricata L of the same dose.
Similarly, A. muricata caused higher percentage inhibition of NO radical than ascorbic acid at all the tested doses. On the contrary, the percentage inhibition of NO radical by K. grandifoliola was lower at the doses of 20 µg/ml and 60 µg/ml but higher at the doses of 80 µg/ml and 100 µg/ml and similar at the dose of 40 µg/ml when compared to ascorbic acid. Comparatively, K. grandifoliola caused lower percentage inhibition of NO radical than A. muricata at the doses of 20 µg/ml, 40 µg/ml, and 60 µg/ml but the same percentage inhibition as A. muricata at the doses of 80 µg/ml and 100 µg/ml, even though the percentage inhibition of both extracts increased in a dose-dependent manner (Figure 2B).
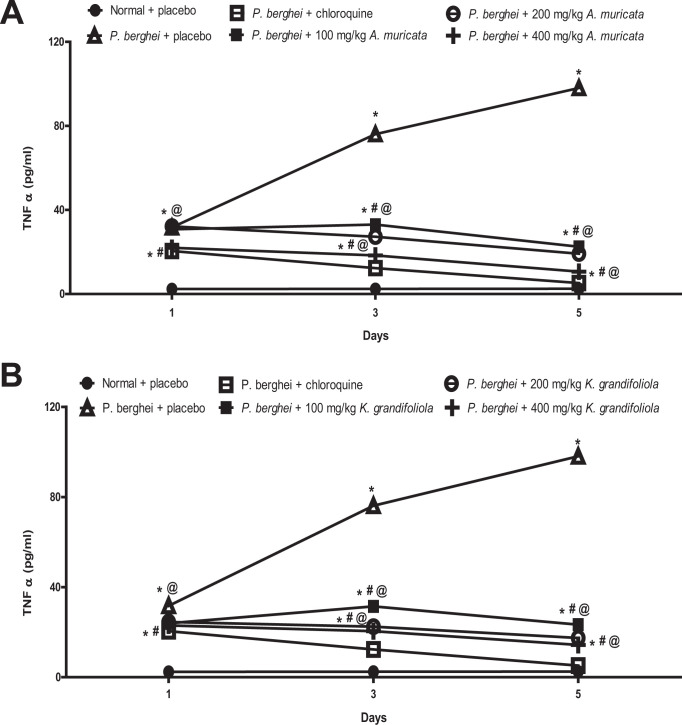
A. muricata and K. grandifoliola Reduced TNF- α in P. berghei-Infected BALB/c Mice
The TNF-α was increased in P. berghei-infected BALB/c mice treated with placebo but reduced in P. berghei-infected BALB/c mice treated with A. muricata, K. grandifoliola, and chloroquine while it does not change in non-infected mice that received placebo. To determine the impacts of A. muricata and K. grandifoliola on the daily TNF-α level, the means in rats that received A. muricata or K. grandifoliola was compared to those that received placebo and chloroquine. It was observed that none of the 3 doses of A. muricata and K. grandifoliola reduced TNF-α level as much as chloroquine (Figure 3).
Figure 3.
A. muricata (A) and K. grandifoliola (B) extracts reduced TNF-α in P. berghei-infected BALB/c mice. *p < 0.05 vs. normal + placebo of the same day; # p < 0.05 vs. P. berghei + placebo of the same day; @ p < 0.05 vs. P. berghei + chloroquine of the same day.
The TNF-α levels in infected mice treated with all doses of A. muricata were higher than the levels in infected mice treated with chloroquine but lower than the level in infected mice treated with placebo throughout the experimental period (Figure 3A).
Similarly, the TNF-α levels in infected mice treated with all doses of K. grandifoliola were higher than the levels in infected mice treated with chloroquine but lower than the level in infected mice treated with placebo throughout the experimental period (Figure 3B).
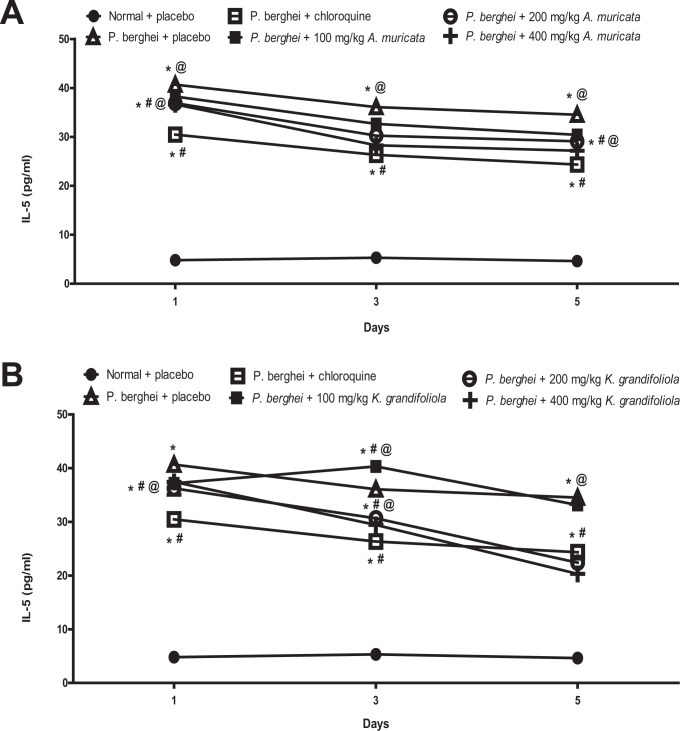
A. muricata and K. grandifoliola Reduced IL-5 in P. berghei-Infected BALB/c Mice
The IL-5 was reduced in P. berghei-infected BALB/c mice treated with placebo, A. muricata, K. grandifoliola, and chloroquine while it did not change in non-infected mice that received placebo.
The IL-5 levels in infected mice treated with all doses of A. muricata were higher than the levels in infected mice treated with chloroquine but lower than the level in infected mice treated with placebo at throughout the observation period (Figure 4A). Similarly, the IL-5 levels in infected mice treated with all doses of K. grandifoliola were higher than the levels in infected mice treated with chloroquine (except those treated with 200 mg/kg and 400 mg/kg for 5 days) but lower than the level in infected mice treated with placebo (Figure 4B).
Figure 4.
A. muricata (A) and K. grandifoliola (B) extracts reduced IL-5 in P. berghei-infected BALB/c mice. *p < 0.05 vs. normal + placebo of the same day; # p < 0.05 vs. P. berghei + placebo of the same day; @ p < 0.05 vs. P. berghei + chloroquine of the same day.
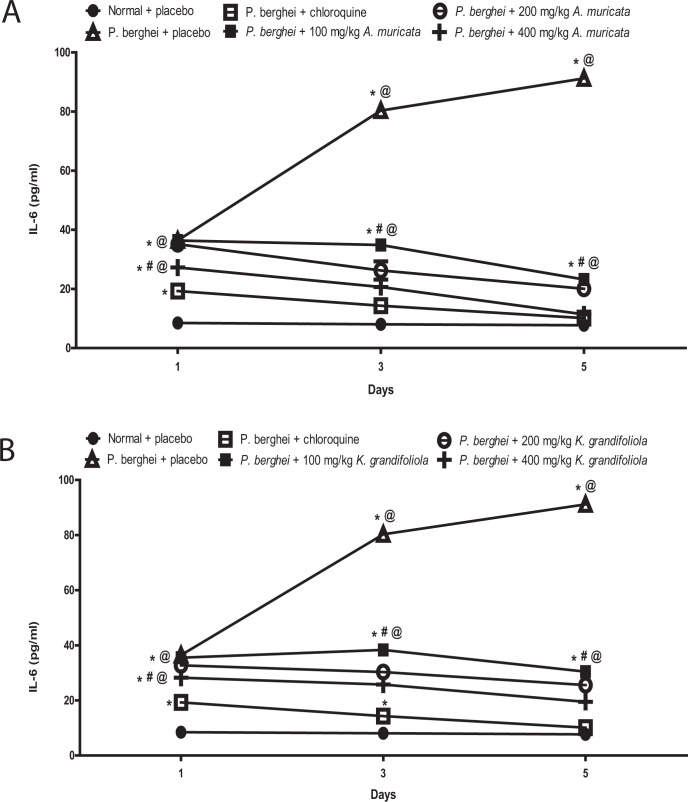
A. muricata and K. grandifoliola Reduced IL-6 in P. berghei-Infected BALB/c Mice
The IL-6 was increased in P. berghei-infected BALB/c mice treated with placebo but reduced in P. berghei-infected BALB/c mice treated with A. muricata, K. grandifoliola, and chloroquine while it does not change in non-infected mice that received placebo. It was observed that none of the 3 doses of A. muricata and K. grandifoliola reduced TNF-α level as much as chloroquine.
The IL-6 levels in infected mice treated with all doses of A. muricata were higher than the levels in infected mice treated with chloroquine but lower than the level in infected mice treated with placebo (Figure 5A). Similarly, the IL-6 levels in infected mice treated with all doses of K. grandifoliola were higher than the levels in infected mice treated with chloroquine but lower than the level in infected mice treated with placebo (Figure 5B).
Figure 5.
A. muricata (A) and K. grandifoliola (B) extracts reduced IL-6 in P. berghei-infected BALB/c mice. *p < 0.05 vs. normal + placebo of the same day; # p < 0.05 vs. P. berghei + placebo of the same day; @ p < 0.05 vs. P. berghei + chloroquine of the same day.
Discussions
The extracts of A. muricata and K. grandifoliola are widely used as folk medicine in Africa due to their ethnomedicinal importance. The present study examined their antiparasitemic effects and explored their cytokine and free radical inhibitory potentials as possible mechanisms of their herbal treatment of CM induced by P. berghei. It was observed that both extracts attenuated the increases in parasitemia and cytokines induced in-vivo by P. berghei. It was also observed that both extracts scavenged the DPPH and NO radicals in-vitro. However, neither A. muricata nor K. grandifoliola caused anti-parasitemia as much as chloroquine, a known antimalarial drug. This is consistent with a previous report that methanol extract of A. muricata inhibits plasmodium parasite in-vitro 40 and in-vivo.25
Is the parasitemia induced by P. berghei related to the generation of free radical and enhancement of inflammatory cytokines? The regulatory role of the CNS on the release of TNF-α and other cytokines by the immune system is well known. Some CNS disorders, including cerebral malaria induced by P. falciparum and P. berghei, are known to modify the release of cytokines in humans and animals. Some clinical features of the CM have been associated with the accumulation of parasitized RBCs in the microvasculature of the brain due to interactions between the over-expressed adhesion molecules (caused by an increase in TNF-α) and parasite proteins. The increased TNF-α release from the host cells to the brain and plasma following exposure to various malarial antigens causes upregulation of adhesion molecule-1 and TNF-α receptors.41 Studies have also shown that TNF-α production is phase-dependent, with the initial CM phase relating to a reduction in parasitic load while the late phase relates to disease severity.42 The dual role of TNF-α led us to estimate its levels on different days of the experimental period. As expected, the TNF-α consistently increased in P. berghei-induced parasitemic mice when compared to the baseline and also to normal mice throughout the experimental period. This supports the contention that P. berghei-induced malaria infection in mice is associated with an increase in TNF-α as reviewed by some authors.43
Are interleukins 5 and 6 affected by P. berghei-induced malaria infection? The IL-6 is a pleiotropic cytokine that can be produced by many cell types, including T cells, monocytes and endothelial cells, all of which are key to the lesion of CM. Since the synthesis and release of IL-6 are induced by the TNF-α, its trend during the experimental period was investigated. Furthermore, thrombocytopenia is a known complication of malaria44 and the role of IL-6 in the production of platelets has been well-documented. For instance, patients with reactive thrombocytosis have been reported to show an increase in IL-6,45 while administration of IL-6 also increases circulating platelets count.46 Though the present study is limited by the non-availability of data on platelets level, our observation that the IL-6 level increased in P. berghei-induced malaria infection is consistent with the report of Raza et al44 which also observed an increase, albeit insignificant, in IL-6 level in P. vivax-associated thrombocytopenic Souther Pakistani population. It was speculated that the increase in IL-6 observed in this study might be associated with the increased TNF-α throughout the experimental period. Our association of increased IL-6 to malaria infection is similar to the previous report of Grau et al47 who showed that IL-6 is produced in considerable amount during blood-stage infection by the malaria parasite, which is involved in hypergammaglobulinemia rather than in the pathogenesis of cerebral complications. Our data are also consistent with previous studies that reported an increase in IL-5 and IL-6 in various kinds of infectious diseases, including malaria.48,49 Thus, the elevation of pro- and anti-inflammatory cytokines is consistent with the previous study of Basir et al30 in P. berghei-treated ICR mice.
Plasmodia digest hemoglobin, which leads to the liberation of heme that will eventually trigger the production of ROS, thus implicating free radical in the pathophysiology of malaria.50 The malaria-induced free radical generation has been reported to cause anemia51 and apoptosis.52 Moreover, the formation of free radical intermediates is the mechanism explored by some antimalarial drugs e.g artemisinin for the destruction of plasmodium parasites.53 The DPPH assay is a commonly employed marker in redox studies of plant extracts or certain compounds within a short period as it provides information on the reactivity of extracts with a stable free radical. On the other hand, a combine USA/Tanzania study has shown that the suppression of plasma NO concentration is involved in the pathogenesis of cerebral malaria, as Tanzanian children with cerebral malaria had decreased plasma and urine nitrogen oxide concentration. Other studies have also shown an inverse relationship between NO and cerebral malaria.54 Is the anti-parasitemia induced by A. muricata and H. grandifoliola associated with their anti-oxidant property?
Malarial infection and its consequences have been substantially ameliorated by plants and compounds that have anti-oxidant property. In fact, animal studies have demonstrated that the development of cerebral complications from malaria can be prevented by anti-oxidants. The anti-oxidant property of a polyherbal anti-malarial product was also reported.55 In our in-vitro study, the free radical-scavenging effect of A. muricata and K. grandifoliola to that of vitamin C, a widely-known anti-oxidant, were compared. It was observed that the A. muricata caused higher percentage inhibition of DPPH radical at 20 µg/ml and 100 µg/ml but the same percentage inhibition of DPPH radical as ascorbic acid at 40 µg/ml, 60 µg/ml and 80 µg/ml, while all the doses caused more inhibition of NO than ascorbic acid. On the contrary, the percentage inhibition of DPPH radical was lower in all the doses of K. grandifoliola while the inhibition of NO was lower in only 20 µg/ml and 60 µg/ml but higher in 80 µg/ml and 100 µg/ml K. grandifoliola when compared to ascorbic acid. Comparatively, K. grandifoliola caused lower percentage inhibition of DPPH and NO radicals than A. muricata. Thus, our data provide in-vitro pieces of evidence that the anti-parasitemic effect of these 2 extracts are mediated by the anti-oxidant mechanism. The comparatively lower anti-oxidant effect of the 2 extracts, when compared to vitamin C, is also consistent with a report that A. muricata’s anti-oxidant property was 1000 times less active than the commercial butylated hydroxytoluene.56
Is the anti-parasitemic effect of A. murucata and K grandifoliola mediated by cytokines? Many natural products, including A. murucata, have been shown to have an immunomodulatory effect with promising anti-inflammatory effect. Having established the fact that P. berghei-induced parasitemia is associated with elevation of cytokines and oxidative stress, it was further investigated if the anti-parasitemic effect of the extracts is associated with cytokines depletion. The plasma levels of TNF-α, IL-5, and IL-6 were all reduced in P. berghei-infected mice treated with the extracts compared to the infected mice treated with placebo, even though none of the extracts was as effective as chloroquine. Our study is consistent with the previous anti-inflammatory report of others where the ethanol extract of A. muricata reduced TNF-α, IL-1β and IL-6.57,58 This is in addition to the modulation of CXCL10 (a strong independent marker of CM) by A. muricata.59
The extract of A. muricata has been reported to contain about 212 identified and isolated secondary metabolites like flavonoids, tannins, saponins, alkaloids, polyphenols, kaempferol, diterpenoids, essential oils, acetogenin compounds and megastigmanes,60 some of which are known to possess anti-plasmodial and antimalarial effects.61 More than 120 Acetogenins, which are the most predominant bioactive compounds, have been reported from the leaves, seeds, stems, fruit peel and pulp of A. muricata, out of which 46 have been identified from the leaves.60 Also, around 22 alkaloids62 and 34 phenolics63 compounds have been identified in the A. muricata leaves. The total phenolic content of A. muricata has been positively correlated with its anti-oxidant potential.64 In this study, it was confirmed that both A. muricata and K. grandifoliola contain flavonoids, saponins, tannins, alkaloids, triterpenoids, reducing sugars, cardiac glycosides and some essential elements including sodium and potassium. Speculatively, the phytochemicals and elements present in both plant extract could have synergistically contributed to the antimalarial, anti-oxidant and anti-inflammatory effects reported herein.
Conclusion
This study shows that the ethanol extracts of A. muricata and K. grandifoliola reduce parasitemia in P. berghei-treated mice BALB/c b scavenging free radicals and reducing cytokines, though the extracts were not as effective as chloroquine.
Footnotes
Authors’ Note: Hope Onohuean conceived, designed, and carried out the study. Hope Onohuean and Abdullateef I. Alagbonsi analyzed and interpreted the data, drafted the manuscript. All authors revised the manuscript, read and make the final corrections. All the authors have read and agreed to the publication of the finding as contained in the manuscript. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The experimental protocol was approved by the KIU-Ethics Committee and carried out in accordance with the guidelines given by the Uganda Council for Higher Education (UCHE).
Declaration of Conflicting Interests: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Hope Onohuean, MSc, PhD Fellow  https://orcid.org/0000-0002-1890-6324
https://orcid.org/0000-0002-1890-6324
Ibe M. Usman, MSc, PhD Fellow  https://orcid.org/0000-0001-6624-1286
https://orcid.org/0000-0001-6624-1286
Keneth K. Iceland, MSc  https://orcid.org/0000-0002-5763-7964
https://orcid.org/0000-0002-5763-7964
Joseph O. C. Ezeonwumelu, MSc, PhD Fellow  https://orcid.org/0000-0002-9701-2942
https://orcid.org/0000-0002-9701-2942
References
- 1.Marchand RP, Culleton R, Maeno Y, Quang NT, Nakazawa S. Co-infections of Plasmodium knowlesi, P. falciparum, and P. vivax among humans and Anopheles dirus mosquitoes, Southern Vietnam. Emerg Infect Dis. 2011;17(7):1232–1239. doi:10.3201/eid1707.101551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sullivan D. Uncertainty in mapping malaria epidemiology: implications for control. Epidemiol Rev. 2010;32(1):175–187. doi:10.1093/epirev/mxq013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Global Status Report on Road Safety 2018; 2018. [Google Scholar]
- 4.Autino B, Noris A, Russo R, Castelli R. Epidemiology of malaria in endemic areas. Mediterr J Hematol Infect Dis. 2012;4(1):e2012060. doi:10.4084/MJHID.2012.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ugandan Ministry of Health. The Uganda Malaria Reduction Strategic Plan 2014-2020, Minist Heal Ugandan; 2014. [Google Scholar]
- 6.FY 2020 Uganda Malaria Operational Plan, Accessed September 10, 2020.www.pmi.gov
- 7.D. for International Development. DFID Uganda Operational Plan 2014; 2014. [Google Scholar]
- 8.White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM. Malaria. Lancet. 2014;383(9918):723–735. doi:10.1016/S0140-6736(13)60024-0 [DOI] [PubMed] [Google Scholar]
- 9.Maccormick IJC, Beare NAV, Taylor TE, et al. Cerebral malaria in children: using the retina to study the brain. Brain. 2014;137(pt 8):2119–2142. doi:10.1093/brain/awu001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bondi FS.The incidence and outcome of neurological abnormalities in childhood cerebral malaria: a long-term follow-up of 62 survivors. Trans R Soc Trop Med Hyg. 1992;86(1):17–19. doi:10.1016/0035-9203(92)90420-h [DOI] [PubMed] [Google Scholar]
- 11.Guo J, Waknine-Grinberg JH, Mitchell AJ, Barenholz Y, Golenser J. Reduction of experimental cerebral malaria and its related proinflammatory responses by the novel liposome-based β-methasone nanodrug, Biomed Res Int. 2014;2014:292471. doi:10.1155/2014/292471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khandare AV, Bobade D, Deval M, Patil T, Saha B, Prakash D. Expression of negative immune regulatory molecules, pro-inflammatory chemokine and cytokines in immunopathology of ECM developing mice. Acta Trop. 2017;172:58–63. doi:10.1016/j.actatropica.2017.04.025 [DOI] [PubMed] [Google Scholar]
- 13.Scholar E. Chloroquine, in: XPharm Compr. Pharmacol Ref. 2007. doi:10.1016/B978-008055232-3.61444-8 [Google Scholar]
- 14.White NJ. Qinghaosu (artemisinin): the price of success. Science. 2008;80. doi:10.1126/science.1155165 [DOI] [PubMed] [Google Scholar]
- 15.Wellems TE, Plowe CV. Chloroquine-resistant malaria. J Infect Dis. 2001;184(6):770–776. doi:10.1086/322858 [DOI] [PubMed] [Google Scholar]
- 16.Shah NK, Dhillon GPS, Dash AP, Arora U, Meshnick UR, Valecha N.Antimalarial drug resistance of Plasmodium falciparum in India: changes over time and space. Lancet Infect Dis. 2011;11(1):57–64. doi:10.1016/S1473-3099(10)70214-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocamora F, Zhu L, Liong KY, et al. Oxidative stress and protein damage responses mediate artemisinin resistance in malaria parasites. PLoS Pathog. 2018;14(3):e1006930. doi:10.1371/journal.ppat.1006930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woodrow CJ, White NJ. The clinical impact of artemisinin resistance in Southeast Asia and the potential for future spread. FEMS Microbiol Rev. 2017.41(1):34–48. doi:10.1093/femsre/fuw037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coria-Téllez AV, Montalvo-Gónzalez E, Yahia EM, Obledo-Vázquez EN. Annona muricata: a comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arab J Chem. 2018;11(5):662–691. doi:10.1016/j.arabjc.2016.01.004 [Google Scholar]
- 20.Foong CP, Hamid RA. Evaluation of anti-infammatory activities of ethanolic extract of Annona muricata leaves. Brazilian J Pharmacogn. 2012;22:1301–1307. doi:10.1590/S0102-695X2012005000096 [Google Scholar]
- 21.Ahalya B, Shankar KR, Kiranmayi GVN. Exploration of anti-hyperglycemic and hypolipidemic activities of ethanolic extract of Annona muricata bark in alloxan induced diabetic rats. Int J Pharm Sci Rev Res. 2014;25(2):21–27. [Google Scholar]
- 22.Yajid AI, Ab Rahman HS, Wong MPK, Wan Zain WZ. Potential benefits of Annona muricata in combating cancer: a review. Malays J Med Sci. 2018;25(1):5–15. doi:10.21315/mjms2018.25.1.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Omara T, Kiprop AK, Ramkat RC, et al. Medicinal plants used in traditional management of cancer in Uganda: a review of ethnobotanical surveys, phytochemistry, and anticancer studies. Evid Based Complement Alternat Med. 2020;2020:3529081. doi:10.1155/2020/3529081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan LTH, Lee LH, Yin WF, et al. Traditional uses, phytochemistry, and bioactivities of Cananga odorata (ylang-ylang), evidence-based complement. Altern Med. 2015;2015:1–30. doi:10.1155/2015/896314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Somsak V, Polwiang N, Chachiyo S. In Vivo antimalarial activity of Annona muricata leaf extract in mice infected with Plasmodium berghei. J Pathog. 2016;2016:3264070. doi:10.1155/2016/3264070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohd Abd Razak MR, Afzan A, Ali R, et al. Effect of selected local medicinal plants on the asexual blood stage of chloroquine resistant Plasmodium falciparum. BMC Complement Altern Med. 2014;14:492. doi:10.1186/1472-6882-14-492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khaya grandifoliola C.DC: A potential source of active ingredients with pharmacological activities. 2019. Accessed September 10, 2020.https://www.longdom.org/proceedings/khaya-grandifoliola-cdc-a-potential-source-of-active-ingredients-with-pharmacological-activities-46549.html
- 28.Khaya grandifoliola (PROTA)—PlantUse English. 2008. Accessed September 10, 2020.https://uses.plantnet-project.org/en/Khaya_grandifoliola_(PROTA)
- 29.Agbedahunsi JM, Elujoba AA, Makinde JM, Oduda AMJ. Antimalarial activity of Khaya grandifoliola stem-bark. Pharm Biol. 1998;36(1):8–12. doi:10.1076/phbi.36.1.8.4613 [Google Scholar]
- 30.Basir R, Rahiman SSF, Hasballah K, et al. Plasmodium berghei ANKA infection in ICR mice as a model of cerebral malaria. Iran J Parasitol. 2012;7(4):62–74. [PMC free article] [PubMed] [Google Scholar]
- 31.Sankeshwari R, Ankola A, Bhat K, Hullatti K.Soxhlet versus cold maceration: which method gives better antimicrobial activity to licorice extract against Streptococcus mutans? J Sci Soc. 2018;45(2):67. 10.4103/jss.jss_27_18 [DOI] [Google Scholar]
- 32.Evans W. Trease and Evans’ pharmacognosy, 13th ed. Bailliere Tindall; 1989. [Google Scholar]
- 33.Charan J, Kantharia N. How to calculate sample size in animal studies? J Pharmacol Pharmacother. 2013;4(4):303. doi:10.4103/0976-500X.119726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braca A, De Tommasi N, Di Bari L, Pizza C, Politi M, Morelli I. Antioxidant principles from Bauhinia tarapotensis. J Nat Prod. 2001;64(7):892–895. doi:10.1021/np0100845 [DOI] [PubMed] [Google Scholar]
- 35.Hope O, Ibrahim OA, Saheed LM, Josiah IE, Ismaila IO. Chloroform seed extract of Buchholzia coriacea (Capparaceae) ameliorates complete Freund’s adjuvant-induced chronic inflammation in rat 1 2 2 3 2 L’extrait de graines de chloroforme de Buchholzia coriacea (Capparaceae) améliore complètement l’inflammation, West African J Pharm. 2018;29:95–104. [Google Scholar]
- 36.Randhawa MA. Calculation of LD50 values from the method of Miller and Tainter, 1944. J Ayub Med Coll Abbottabad. 2009;21(3):184–185. Accessed September 19, 2020. http://europepmc.org/article/MED/20929045 [PubMed] [Google Scholar]
- 37.Virginia Tech. SOP: Blood Collection in the Mouse. Tail Vein; 2017. [Google Scholar]
- 38.Yang H, Wu C, Liu F, et al. Blood collection through subclavian vein puncture in mice. J Vis Exp. 2019:147. doi:10.3791/59556 [DOI] [PubMed] [Google Scholar]
- 39.(PDF) Etude De Toxicité Aiguë De L’extrait Total Aqueux De Phyllanthus Amarus (Schum & Thonn) Chez Les Souris. 2005. Accessed September 28, 2020.https://www.researchgate.net/publication/268982878_ETUDE_DE_TOXICITE_AIGUE_DE_L’EXTRAIT_TOTAL_AQUEUX_DE_PHYLLANTHUS_AMARUS_SCHUM_THONN_CHEZ_LES_SOURIS
- 40.Boyom FF, Fokou PVT, Yamthe LRT, et al. Potent antiplasmodial extracts from Cameroonian Annonaceae. J Ethnopharmacol. 2011;134(3):717–724. doi:10.1016/j.jep.2011.01.020 [DOI] [PubMed] [Google Scholar]
- 41.Gimenez F, Barraud de Lagerie S, Fernandez C, Pino P, Mazier D. Tumor necrosis factor alpha in the pathogenesis of cerebral malaria. Cell Mol Life Sci. 2003;60(8):1623–1635. doi:10.1007/s00018-003-2347-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Omer FM, Kurtzhals JA, Riley EM. Maintaining the immunological balance in parasitic infections: a role for TGF-beta? Parasitol Today. 2000;16(1):18–23. doi:10.1016/s0169-4758(99)01562-8 [DOI] [PubMed] [Google Scholar]
- 43.Leão L, Puty B, Dolabela MF, et al. Association of cerebral malaria and TNF-α levels: a systematic review. BMC Infect Dis. 2020;20(1):442. doi:10.1186/s12879-020-05107-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raza A, Khan MS, Ghanchi NK, Raheem A, Beg MA, Tumour necrosis factor, interleukin-6 and interleukin-10 are possibly involved in Plasmodium vivax-associated thrombocytopaenia in southern Pakistani population. Malar J. 2014;13:323. doi:10.1186/1475-2875-13-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heits F, Stahl M, Ludwig D, Stange EF, Jelkmann W. Elevated serum thrombopoietin and interleukin-6 concentrations in thrombocytosis associated with inflammatory bowel disease. J Interferon Cytokine Res. 1999;19(7):757–760. doi:10.1089/107999099313604 [DOI] [PubMed] [Google Scholar]
- 46.Veldhuis GJ, Willemse PHB, Sleijfer DT, et al. Toxicity and efficacy of escalating dosages of recombinant human interleukin-6 after chemotherapy in patients with breast cancer or non-small-cell lung cancer. J Clin Oncol. 1995;13(10):2585–2593. doi:10.1200/JCO.1995.13.10.2585 [DOI] [PubMed] [Google Scholar]
- 47.Grau GE, Frei K, Piguet PF, et al. Interleukin 6 production in experimental cerebral malaria: modulation by anticytokine antibodies and possible role in hypergammaglobulinemia. J Exp Med. 1990;172(5):1505–1508. doi:10.1084/jem.172.5.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nmorsi O, Isaac C, Ukwandu N, Ohaneme B. Pro–and anti–inflammatory cytokines profiles among Nigerian children infected with Plasmodium falciparum malaria. Asian Pac J Trop Med. 2010;3(1):41–44. doi:10.1016/S1995-7645(10)60029-6 [Google Scholar]
- 49.Prakash D, Fesel C, Jain R, Cazenave PA, Mishra GC, Pied S. Clusters of cytokines determine malaria severity in Plasmodium falciparum-infected patients from endemic areas of Central India. J Infect Dis. 2006;194(2):198–207. doi:10.1086/504720 [DOI] [PubMed] [Google Scholar]
- 50.Becker K, Tilley L, Vennerstrom JL, Roberts D, Rogerson S, Ginsburg H. Oxidative stress in malaria parasite-infected erythrocytes: host–parasite interactions. Int J Parasitol. 2004;34(2):163–189. doi:10.1016/j.ijpara.2003.09.011 [DOI] [PubMed] [Google Scholar]
- 51.Kremsner P, Greve B, Lell B, Luckner D, Schmid D. Malarial anaemia in African children associated with high oxygen-radical production. Lancet. 2000;355(9197):40–41. doi:10.1016/S0140-6736(99)04761-3 [DOI] [PubMed] [Google Scholar]
- 52.Guha M, Kumar S, Choubey V, Maity P, Bandyopadhyay U. Apoptosis in liver during malaria: role of oxidative stress and implication of mitochondrial pathway. FASEB J. 2006;20(8):1224–1226. doi:10.1096/fj.05-5338fje [DOI] [PubMed] [Google Scholar]
- 53.Zhang S, Chen H, Gerhard GS. Heme synthesis increases artemisinin-induced radical formation and cytotoxicity that can be suppressed by superoxide scavengers. Chem Biol Interact. 2010;186(1):30–35. doi:10.1016/j.cbi.2010.03.021 [DOI] [PubMed] [Google Scholar]
- 54.Eisenhut M. The evidence for a role of vasospasm in the pathogenesis of cerebral malaria. Malar J. 2015;14:405. doi:10.1186/s12936-015-0928-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arrey Tarkang P, Nwachiban Atchan AP, Kuiate JR, Okalebo FA, Guantai AN, Agbor GA. Antioxidant potential of a polyherbal antimalarial as an indicator of its therapeutic value. Adv Pharmacol Sci. 2013;2013:678458. doi:10.1155/2013/678458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alitonou G, Tchobo F, Sessou P, Avlessi F. Chemical composition, antiradical and antiinflammatory activities of four Annonaceae from Benin. Int J Pharm Chem Biol Sci. 2011;3:914–923. [Google Scholar]
- 57.Laksmitawati DR, Prasanti AP, Larasinta N, et al. Widowati, anti-inflammatory potential of Gandarusa (Gendarussa vulgaris Nees) and soursoup (Annona muricata L) extracts in LPS stimulated-macrophage cell (RAW264.7). J Nat Remedies. 2016;16(3):73. doi:10.18311/jnr/2016/5367 [Google Scholar]
- 58.Chan P, Ah R, Mh K, A Z, Anti-arthritic activities of Annona muricata L. leaves extract on complete Freund’s adjuvant (CFA)—induced arthritis in rats. Planta Med. 2010;76(12):166. doi:10.1055/s-0030-1264464 [Google Scholar]
- 59.Djamiatun K, Matug SMA, Prasetyo A, Wijayahadi N, Nugroho D. Annona muricata modulate brain-CXCL10 expression during cerebral malaria phase. IOP Conf Ser Earth Environ Sci. 2017;55:012034. doi:10.1088/1755-1315/55/1/012034 [Google Scholar]
- 60.Abdul Wahab SM, Jantan I, Haque MA, Arshad L. Exploring the leaves of Annona muricata L. as a source of potential anti-inflammatory and anticancer agents. Front Pharmacol. 2018;9:661. doi:10.3389/fphar.2018.00661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pimenta LPS, Garcia GM, Gonçalves SGDV, Dionísio BL, Braga EM, Mosqueira VCF. In vivo antimalarial efficacy of acetogenins, alkaloids and flavonoids enriched fractions from Annona crassiflora mart. Nat Prod Res. 2014;28(16):1254–1259. doi:10.1080/14786419.2014.900496 [DOI] [PubMed] [Google Scholar]
- 62.Fofana S, Keita A, Balde S, Ziyaev R, Aripova SF. Alkaloids from leaves of Annona muricata. Chem Nat Compd. 2012;48(15):714–714. doi:10.1007/s10600-012-0363-5 [Google Scholar]
- 63.George VC, Kumar DRN, Suresh PK, Kumar RA. Antioxidant, DNA protective efficacy and HPLC analysis of Annona muricata (soursop) extracts. J Food Sci Technol. 2015;52(4):2328–2335. doi:10.1007/s13197-014-1289-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gavamukulya Y, Abou-Elella F, Wamunyokoli F, AEl-Shemy H. Phytochemical screening, anti-oxidant activity and in vitro anticancer potential of ethanolic and water leaves extracts of Annona muricata (Graviola). Asian Pac J Trop Med. 2014;7: S355–S363. 10.1016/S1995-7645(14)60258-3 [DOI] [PubMed] [Google Scholar]