Figure 4: Transient knockdown of A2B1 enhanced antiestrogen-sensitivity of LCC9, LY2, and T47D cells.
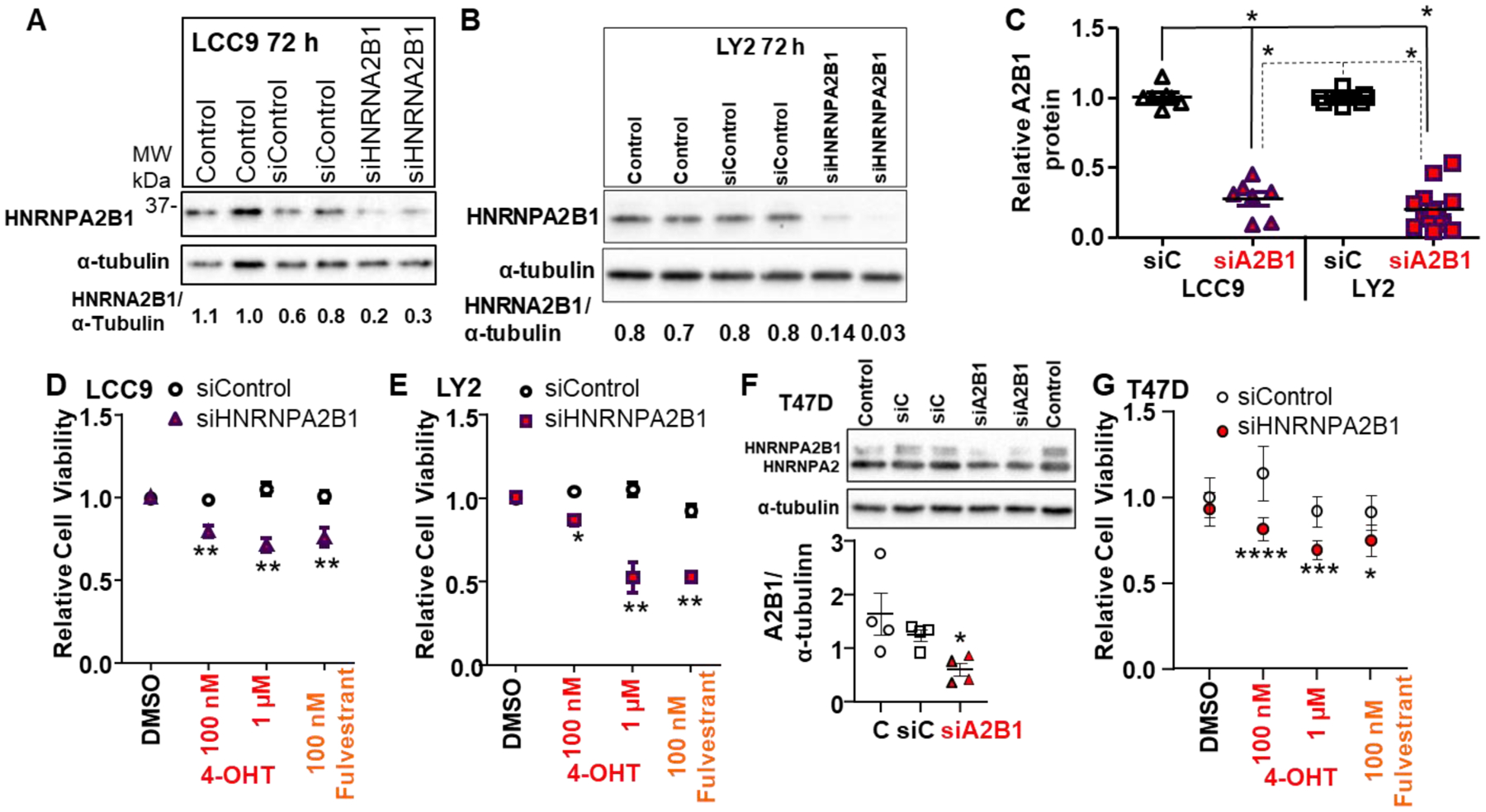
For A,B, and F the indicated cell lines were either not transfected (Control) or were transfected with siControl or siA2B1 for 48 h in Opti-MEM prior to 36 h in SS medium. For the blots shown, 30 μg WCE was loaded/lane. C: Summary of western blots of A2B1 relative to α-tubulin in LCC9 (n = 7) and LY2 (n = 13) separate WCE. *p < 0.0001 for one-way ANOVA followed by Tukey’s post hoc multiple comparisons test. D, E, and G summarize 8 (LCC9), 10 (LY2), and 4 (T47D) separate, independent MTT assays. After transfection and ~ 16 h in OPTI-MEM, cells were incubated for an additional ~ 32 h in SS medium prior to treatment with 100 nM or 1 μM 4-OHT or 100 nM fulvestrant, with medium changed every 48h, for 4 d. Values are the relative mean viability compared DMSO (vehicle control) ± SEM. *p < 0.05, ** < 0.01,***p < 0.001,***p < 0.0001 versus siControl DMSO-treated cells in two-way ANOVA, Tukey’s multiple comparison post hoc test. F shows a representative western blot for A2B1 knockdown in T47D cells using a monoclonal antibody that recognizes both the B1 and A2 splice variants of HNRNPA2B1. The graph shows data from 4 experiments, *p = 0.039 in one-way ANOVA followed by Tukey’s post hoc test.
