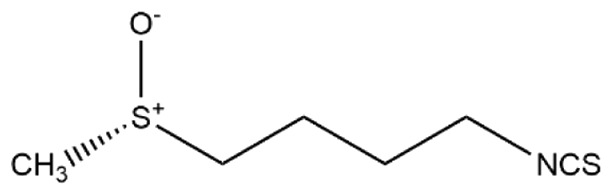
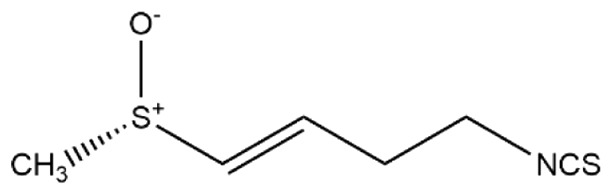
Table I.
Differences between sulforaphane and sulforaphene.
| Property | Sulforaphane | Sulforaphene |
|---|---|---|
| Molecular formula | C6H11NOS2 | C6H9NOS2 |
| Structural formula |
|
|
| Chemical name | 1-isothiocyanato-4-[(S)-methylsulfinyl] butane | (E)-4-isothiocyanato-1-methylsulfinylbut-1-ene |
| Molecular mass, g/mol | 177.3 | 175.264 |
| SMILES | C[S@](=O)CCCCN=C=S | CS(=O)C=CCCN=C=S |
| CAS Number | 155320-20-0 | 592-95-0 |
| Purity, % | >98 | >98 |
| Form/State | Solid | Solid |
| Solubility | Soluble in water, in ethanol and in DMSO | Soluble in water |
| XLogP3 | 1.4 | 1.5 |
| Number of hydrogen bond donors | 0 | 0 |
| Number of hydrogen bond acceptors | 4 | 4 |
| Number of rotatable bonds | 5 | 4 |
| Source | Brassica oleracea | Brassica oleracea |
| Biological characterization | Non-competitive antagonist of the aryl hydrocarbon receptor, natural isothiocyanate, antitumor agent, active in vivo and in vitro (https://www.abcam.cn/s-sulforaphane-ah-receptor-antagonist-ab141970.html) | Carcinogenesis inhibitor, anticancer, antidiabetic and antioxidant effects, active in vivo and in vitro (https://www.abcam.cn/s-sulforaphene-carcinogenesis-inhibitor-ab141972.html) |
| Type of anti-cancer effect | Promyelocytic leukemia (1), skin cancer (2), bladder cancer (3), prostate cancer (4), colon cancer (5), pancreatic cancer (6), liver cancer (7), lung cancer (8), nasopharyngeal cancer (9), ovarian cancer (10), breast cancer (11), cervical cancer (12) | Lung cancer (21), esophageal cancer (20), colon cancer (22), gastric cancer (23), liver cancer (24), breast cancer (19), cervical cancer (25), thyroid cancer (26) |
CAS, Chemical Abstracts Service; SMILES, simplified molecular-input line-entry system.
