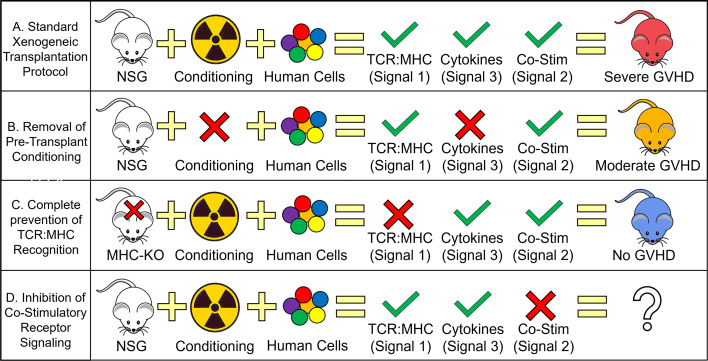
Figure 1.
Human T cell Requirements for GVHD Development During Xenogeneic Transplantation. Schematic depicting the relative contribution of each T cell activation signal toward the development of GVHD. (A) Standard xenogeneic transplant protocols provide all three T cell activation signals, human TCR to murine MHC recognition, pro-inflammatory cytokine secretion from genotoxic conditioning (i.e. γ-irradiation) and human CD28 to murine B7 cross-reactivity (with possible contributions from other co-stimulatory proteins) to cause severe GVHD. (B) Removing the presence of pro-inflammatory cytokines by not conditioning NSG mice prior to transplant results in only a slight decrease in GVHD severity with clinical data using tocilizumab/ruxolitinib also showing modest effects on GVHD mitigation. (C) Complete prevention of human TCR recognition of murine MHC (by knocking out murine MHC) eliminates all signs of GVHD. The widespread adoption of calcineurin inhibitors (e.g. tacrolimus) for GVHD prophylaxis also supports the important role of TCR : MHC interactions though in the case of clinical calcineurin inhibitors, only a partial inhibition is achieved. (D) Blocking co-stimulatory signaling remains the only T cell activation signal not investigated with xenogeneic transplant studies and is only recently entered the clinical domain. Severe GVHD is generally described as achieving ≥ 70% lethality with 3E6 PB-MNC with moderate GVHD ranging from 30-70% lethality with the same dose of human cells.