FIGURE 3.
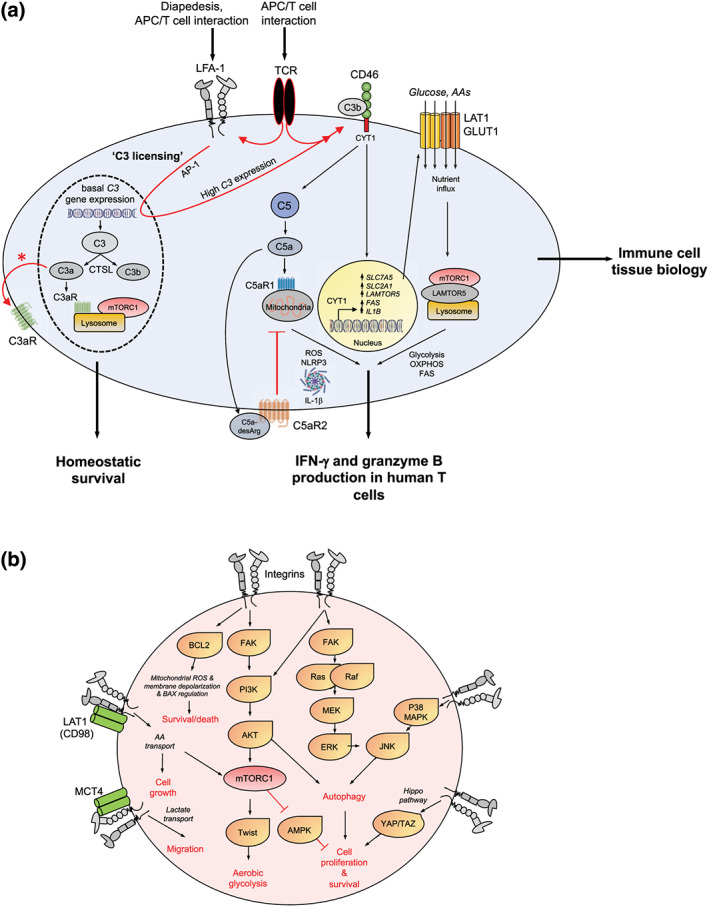
LFA‐1‐induced C3 licensing drives metabolic reprogramming in activated T‐cells. (a) Resting human CD4+ and CD8+ T‐cells sustain basal levels of C3 gene expression and generate continuously C3a and C3b via CTSL cleavage. Cell‐autonomous C3a stimulates the lysosomal C3aR that in turn sustains tonic mTORC1 activation needed for homeostatic survival. Diapedesis or the cognate APC/T‐cell interaction engages T‐cell expressed LFA‐1 (via ICAM‐1) and induces increased C3 gene expression and protein generation—C3 licensing. Timely incoming TCR signals are required to mediate increased intrinsic C3 activation (TCR signals also further activate LFA‐1), rapid surface translocation of C3a and the C3aR (denoted by an asterisk) and of C3b that then induces autocrine CD46 stimulation. CD46 downstream signals include the cleavage and nuclear translocation of its intracellular CYT‐1 domain that drives expression of nutrient transporters GLUT1 (glucose), LAT1 (amino acids, AA), fatty acid synthase or fatty acid synthesis (FAS), and the lysosomal scaffolder LAMTOR5. Together, these events support mTORC1 assembly at the lysosomes and high glycolysis, fatty acid synthesis, OXPHOS, and tricarboxylic acid cycle. CD46 also induces activation of intracellular C5 with C5a engaging the intracellular C5aR1 to produce ROS, NLRP3 inflammasome activation, and intrinsic active IL‐1β that sustains Th1 responses. Surface C5aR2 is a negative regulator of Th1 induction. The CD46/IL‐2R‐driven signals that co‐induce IL‐10 and Th1 contraction are not depicted here. Transmigration‐induced expression of a cell‐autonomous complosome is a defining feature of immune cells in tissue and also needed for normal monocyte/macrophage tissue activity (not shown). (b) Cell metabolic events known to be engaged by different integrins in a range of cells and their outcome on cell survival, proliferation, and migration (see text for details)
