Abstract
Introduction
Preterm birth is a major cause of perinatal morbidity and mortality worldwide. In many countries preterm birth rates are increasing, largely as a result of increases in iatrogenic preterm birth, whereas in other countries rates are stable or even declining. The objective of the study is to describe trends in singleton preterm births in Victoria from 2007 to 2017 in relation to trends in perinatal mortality to identify opportunities for improvements in clinical care.
Material and methods
We conducted a consecutive cross‐sectional study in all women with a singleton pregnancy giving birth at ≥20 weeks of pregnancy in Victoria, Australia, between 2007 and 2017, inclusive. Rates of preterm birth and perinatal mortality were calculated and trends were analyzed in all pregnancies, in pregnancies complicated by fetal growth problems, hypertension, (pre)eclampsia or prelabor rupture of membranes (PROM), and in (low‐risk) pregnancies not complicated by any of these conditions.
Results
There were 811 534 singleton births between 2007 and 2017. Preterm birth increased from 5.9% (4074 births) to 6.4% (4893 births; P < .001), due to an increase in iatrogenic preterm birth from 2.5% (1730 births) to 3.6% (2730 births; P < .001). Comparable trends were seen in pregnancies complicated by fetal growth problems and hypertension and in pregnancies not complicated by small for gestational age (SGA), hypertension, (pre)eclampsia or PROM (all P < .001). In pregnancies complicated by SGA, hypertension, (pre)eclampsia or PROM the perinatal mortality rate from 20 weeks of gestation fell (13 to 12 per 1000 births; P < .001). In pregnancies not complicated by SGA, hypertension, (pre)eclampsia or PROM there was no significant change in the perinatal mortality from 28 weeks and no decrease in the preterm weekly prospective stillbirth risk.
Conclusions
The singleton preterm birth rate in Victoria is increasing, driven by an increase in iatrogenic preterm birth, both in pregnancies complicated by SGA and hypertension, and in pregnancies not complicated by SGA, hypertension, (pre)eclampsia or PROM. While perinatal mortality decreased in the pregnancies complicated by SGA, hypertension, (pre)eclampsia or PROM, no significant reduction in perinatal mortality from 28 weeks or in preterm weekly prospective stillbirth risk was noted in the pregnancies not complicated by any of these conditions.
Keywords: hypertensive disorders in pregnancy, iatrogenic preterm birth, induction of labor, perinatal morbidity and mortality, preterm birth, preterm prelabor rupture of membranes, small for gestational age, spontaneous preterm birth
Abbreviations
- CS
cesarean section
- IOL
induction of labor
- PROM
prelabor rupture of membranes
- SGA
small for gestational age
Key message.
Singleton preterm birth in Victoria increased from 5.9% (2007) to 6.4% (2017), due to an increase in iatrogenic preterm birth (from 2.5% to 3.6%). In high‐risk pregnancies, perinatal mortality rate fell, but it did not fall in low‐risk pregnancies from 28 weeks.
1. INTRODUCTION
Preterm birth remains a major obstetric challenge. It is associated with both short‐ and long‐term complications for the neonate, including death, respiratory problems and poorer neurodevelopment.1, 2, 3, 4 Although risks are highest in early preterm birth (<34+0 weeks of gestation), infants born late preterm (34+0 to 36+6 weeks of gestation) remain at higher risk than their peers born at term.5
Worldwide, every year 15 million babies (11%) are born preterm.1 About 80% of all preterm births occur in singleton pregnancies.6, 7 In high‐income countries preterm birth rates vary between 5% and 13%.6, 7, 8 In many countries rates are increasing, largely due to increases in iatrogenic preterm birth. In others, however, rates are stable or even declining.6, 9, 10 A recent study from South Australia showed that the singleton preterm birth rate increased from 5.1% in 1986 to 7.1% in 2014, with iatrogenic preterm birth accounting for 80% of this increase.11 The preterm birth rate in Victoria in all pregnancies was 8.5% in 2017.12
The aim of this study is to analyze trends in singleton preterm birth in Victoria in relation to trends in perinatal outcome, as we did earlier for twin pregnancies.13 Simultaneous evaluation of these trends is essential, because although iatrogenic birth aims to prevent adverse maternal and perinatal outcome, overtreatment poses a risk. We sought to identify possible driver(s) for change and opportunities to improve clinical care.13
2. MATERIAL AND METHODS
We conducted a consecutive cross‐sectional study among women with a singleton pregnancy who gave birth in Victoria between 2007 and 2017, inclusive. We derived all data from the validated Victorian Perinatal Data Collection,which holds data on all births in Victoria at ≥20 weeks of gestation or, if gestation is unknown, with a birthweight ≥400 grams.14
Annual rates of spontaneous, iatrogenic, early (<34+0 weeks) and late (34+0‐36+6 weeks) preterm birth in liveborn singletons were calculated, as well as rates of preterm induction of labor (IOL) and preterm prelabor cesarean section (CS). Iatrogenic birth was defined as birth caused by medical intervention (either IOL or prelabor CS), and spontaneous birth as labor commencing naturally without any intervention. Cases were excluded if gestational age at delivery or labor type were unknown. Subgroup analyses were performed for four types of pregnancy complications: small for gestational age (SGA; birthweight <10th centile for gestation and sex in the Australian population15), hypertension, (pre)eclampsia and prelabor rupture of membranes (PROM), as well as for pregnancies without any of these complications. International Classification of Diseases 10th Revision Australian Modification codes were used to identify hypertension (O10, O13, O16), (pre)eclampsia (O11, O14‐15) and PROM (O42, P01.1). Pregnancies complicated by hypertension include unspecified, gestational and preexisting hypertension. (Pre)eclampsia includes all forms of preeclampsia, superimposed preeclampsia, HELLP (hemolysis, elevated liver enzymes and low platelet count) syndrome and eclampsia.
We analyzed trends in baseline characteristics including maternal age, parity, body mass index, smoking status and maternal region of birth. Maternal country of birth, classified into geographical regions according to the Standard Australian Classification of Countries, was used as a surrogate for maternal ethnicity, as previously described in detail.16
Trends in adverse pregnancy outcomes were assessed using stillbirth and neonatal death rates and average weekly prospective stillbirth risks. We chose to report stillbirth, neonatal mortality and perinatal mortality in all ongoing pregnancies from 20 weeks (according to the Australian definition), from 24 weeks (excluding extreme preterm births <24 weeks that are not always actively managed), from 28 weeks (as recommended by the World Health Organization to allow international comparison), from 34 weeks (to investigate trends among pregnancies delivered late preterm and term) and from 37 weeks (to investigate trends among pregnancies delivered at term). Annual average weekly prospective risks of stillbirth were calculated for 20+0‐27+6, 28+0‐33+6, 34+0‐36+6 and 37+0‐41+6 weeks using a “fetus‐at‐risk” approach, dividing the number of stillbirths in that gestational age period by the number of women at risk in the same period and expressed as the average weekly risk by further dividing it by the number of weeks in the period.13, 17
2.1. Statistical analyses
Trends in preterm birth, pregnancy complications, maternal characteristics and perinatal mortality were assessed using a chi‐squared test for linear trend. The significance level was set at a probability value of <0.01 based on the large sample size and the desired strength of evidence. Perinatal mortality graphs were produced using a 3‐year‐moving average. Data were described and analyzed using IBM SPSS Statistics 25 (IBM Corp.).
2.2. Ethical approval
Permission to access and analyze data was granted by the Consultative Council on Obstetric and Paediatric Mortality and Morbidity, Safer Care Victoria. Ethical approval for this project was granted by the Monash University Human Research Ethics Committee (Project ID 14414; final amendment approved 8 October 2019).13
3. RESULTS
From 2007 to 2017 there were 812 255 singleton births in Victoria. After exclusion of pregnancies with unknown gestational age at delivery or unknown labor type (spontaneous, iatrogenic), 811 534 (99.9%) remained for analysis, of which 807 885 (99.6%) women had a livebirth and 3649 (0.4%) had a stillbirth.
3.1. Baseline characteristics
Table 1 summarizes demographic changes across the study period. Apart from changes in maternal country of birth there were no major changes in maternal characteristics. Significant upward trends were noted in underweight, normal weight, nulliparity and the number of women of Aboriginal or Torres Strait Islander origin, but the overall changes were very small. Similarly, very small but statistically significant downward trends were seen for maternal age <20 and ≥35 years, smoking, overweight and obesity.
TABLE 1.
Maternal characteristics of all singleton pregnancies in Victoria between 2007 and 2017
| 2007‐2009 | 2010‐2014 | 2015‐2017 |
Trend 2007‐2017a |
||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | P value | |
| All mothers | 209 349 | 370 302 | 231 883 | ||||
| Maternal age (years) | |||||||
| <20 | 7532 | 3.6 | 13 720 | 3.7 | 6120 | 2.6 | <.001 |
| 20‐34 | 146 096 | 69.8 | 268 822 | 72.6 | 169 419 | 73.1 | <.001 |
| ≥35 | 58 651 | 28.0 | 92.799 | 25.1 | 58 977 | 25.4 | <.001 |
| Parity | |||||||
| Nulliparous | 90 186 | 43.1 | 164 932 | 44.5 | 101 317 | 43.7 | <.001 |
| Region of birth | |||||||
| Oceania (Australia and New Zealand) | 156 669 | 75.9 | 256 512 | 69.8 | 150 195 | 65.1 | <.001 |
| Asia | 27 128 | 13.1 | 68 876 | 18.7 | 52 860 | 22.9 | <.001 |
| Europe | 11 293 | 5.5 | 18 138 | 4.9 | 10 897 | 4.7 | <.001 |
| North Africa and the Middle East | 6216 | 3.0 | 11 757 | 3.2 | 8227 | 3.6 | <.001 |
| Americas | 2499 | 1.2 | 4833 | 1.3 | 3244 | 1.4 | <.001 |
| Sub‐Saharan Africa | 2510 | 1.2 | 7474 | 2.0 | 5128 | 2.2 | <.001 |
|
Indigenous status Aboriginal or Torres Strait Islander |
2204 | 1.1 | 4687 | 1.3 | 3324 | 1.4 | <.001 |
| Smokingb | |||||||
| Before 20 weeks of gestation | 8021 | 11.7 | 40 082 | 11.0 | 20 327 | 8.9 | <.001 |
| At or above 20 weeks of gestation | — | — | 20 203 | 5.8 | 12 314 | 5.6 | <.001 |
| BMI (kg/m2)b | |||||||
| BMI <18.5 | 1787 | 2.9 | 10 130 | 3.1 | 7170 | 3.1 | .005 |
| BMI 18.5‐24.9 | 30 911 | 49.8 | 166 478 | 50.6 | 116 358 | 51.0 | <.001 |
| BMI ≥25.0 | 29 414 | 47.4 | 152 243 | 46.3 | 104 410 | 45.8 | <.001 |
| BMI ≥30.0 | 12 483 | 20.1 | 64 644 | 19.7 | 44 310 | 19.4 | .009 |
Trends were analyzed using a chi‐squared test for linear trend.
For smoking <20 weeks of gestation and BMI no data were available for 2007‐2008; for smoking ≥20 weeks of gestation no data were available for 2007‐2009.
The proportion of pregnancies complicated by SGA decreased from 9.5% (6583 births) to 8.6% (6636 births; P < .001), whereas the proportion affected by hypertension (3.3% vs 3.1%; P = .12) and (pre)eclampsia (2.3% vs 2.2%; P = .003) remained relatively stable over time. The incidence of PROM decreased from 8.7% (6018 births) in 2007 to 6.0% (4172 births) in 2009, after which it increased to 9.0% in 2017 (6874 births; P < .001; Supporting Information Figure S1).
3.2. Preterm birth
Figure 1 and Supporting Information Table S1 show the trends in preterm birth in all singletons and in different subgroups. The rate of preterm birth in all singletons increased by 8% from 5.9% (4074 births) in 2007 to 6.4% (4893 births) in 2017 (P < .001). The majority of this increase occurred from 2013 onwards. The increase in preterm birth rate was accounted for by an increase in the rate of late preterm birth, from 4.4% (3028 births) to 4.9% (3776 births; P < .001), whereas the rate of early preterm birth remained stable (1.5% to 1.5%; P = .86). The proportion of spontaneous preterm birth decreased from 3.4% (2344 births) to 2.8% (2163 births; P < .001), but iatrogenic preterm birth increased from 2.5% (1730 births) to 3.6% (2730 births; P < .001). Among early preterm births, a small decrease was seen in spontaneous onset from 0.9% (599 births) to 0.7% (569 births; P < .001), whereas iatrogenic early preterm birth increased slightly from 0.6% (447 births) to 0.7% (548 births; P < .001). Among late preterm births, a larger decrease in spontaneous onset was seen from 2.5% (1745 births) to 2.1% (1594 births; P < .001), as well as an increase in iatrogenic birth from 1.9% (1283 births) to 2.8% (2182 births; P < .001). The rates of both preterm IOL (0.9% to 1.2%; P < .001) and preterm CS (1.6% to 2.3%; P < .001) among all liveborn singletons increased.
FIGURE 1.
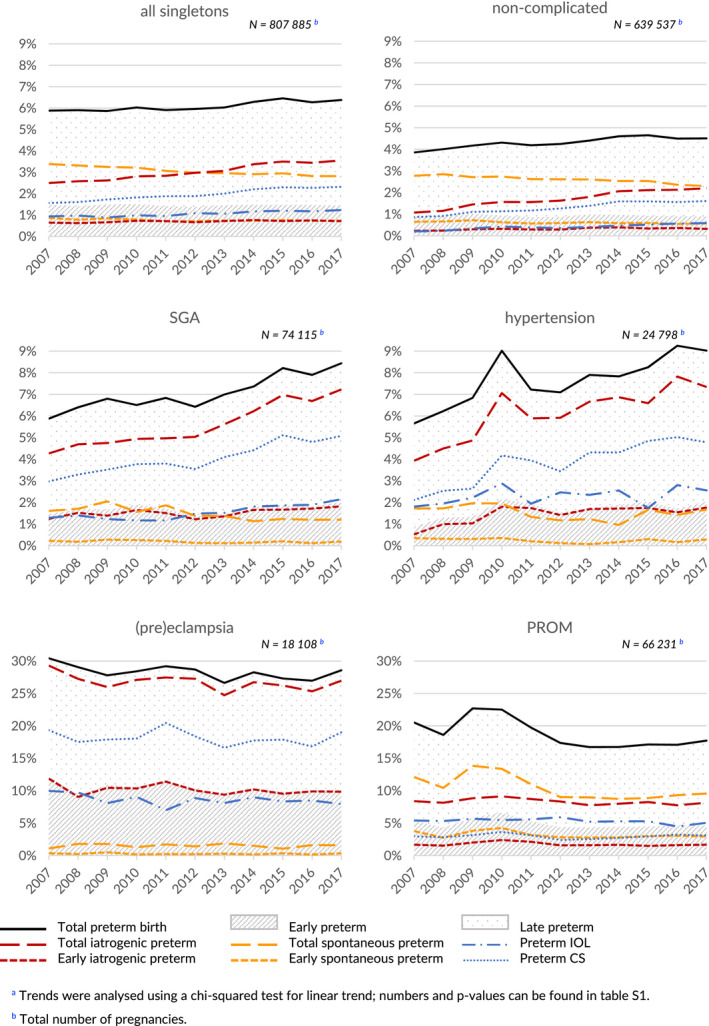
Preterm birth in all liveborn singleton pregnancies, liveborn singleton pregnancies complicated by small for gestational age (SGA), hypertension, (pre)eclampsia or prelabor rupture of membranes (PROM), and liveborn singleton pregnancies not complicated by any of these conditions (non‐complicated): Victoria, 2007‐2017a [Color figure can be viewed at wileyonlinelibrary.com]
In pregnancies complicated by SGA or hypertension comparable but more profound trends of increasing preterm birth were noted. In pregnancies complicated by SGA the total preterm birth rate increased from 5.8% (387 births) to 8.4% (560 births; P < .001), driven by an increase in iatrogenic preterm birth from 4.3% (281 births) to 7.2% (480 births; P < .001). Spontaneous preterm birth decreased slightly in pregnancies complicated by SGA from 1.6% (106 births) to 1.2% (80 births; P < .001). The largest part of the increase in iatrogenic preterm birth was seen from 34 weeks of gestation, although a small significant increase was noted before 34 weeks. Both the rate of preterm IOL (from 1.3% to 2.2%; P = .002) and preterm CS (from 3.0% to 5.1%; P < .001) increased.
In pregnancies complicated by hypertension total preterm birth increased from 5.7% (128 births) to 9.0% (215 births; P < .001), driven by an increase in iatrogenic preterm birth from 3.9% (89 births) to 7.3% (175 births; P < .001). The increase in iatrogenic preterm birth occurred both before 34 weeks of gestation and after (both P < .001). Spontaneous preterm birth was relatively stable (1.7% to 1.7%; P = .119). The rate of preterm IOL was relatively stable (1.8% to 2.6%; P = .152), but the preterm CS rate increased significantly (2.1% to 4.8%; P < .001).
In pregnancies complicated by (pre)eclampsia a downward trend in preterm birth was seen from 30.4% (494 births) to 28.6% (481 births; P = .06), driven by a comparable decrease in iatrogenic preterm birth (P = .05), but these trends were not statistically significant. Spontaneous preterm birth was stable (1.1% to 1.6%; P = .955). No significant changes were seen in the rate of preterm IOL (10.0% to 8.0%; P = .30) and preterm CS (19.3% to 19.0%; P = .43).
In pregnancies complicated by PROM, the preterm birth rate decreased (P < .001), driven by a decrease in spontaneous preterm birth both before and from 34 weeks of gestation (all P ≤ .001), whereas the iatrogenic preterm birth rate was stable (P = .06). The rates of preterm IOL (5.4% to 5.1%; P = .02) and preterm CS (3.0% to 3.1%; P < .001) were relatively stable.
In pregnancies not complicated by SGA, hypertension, (pre)eclampsia or PROM, the preterm birth rate increased from 3.9% (2080 births) to 4.5% (2733 births; P < .001). Spontaneous preterm birth rates in these pregnancies decreased from 2.8% (1499 births) to 2.3% (1398 births; P < .001) whereas iatrogenic preterm birth increased from 1.1% (581 births) to 2.2% (1335 births; P < .001). Most of the changes in iatrogenic and preterm birth occurred in the late preterm phase (both P < .001), but the trends were also observed before 34 weeks of gestation (both P < .001). Both the rate of preterm IOL (0.2% to 0.6%; P < .001) and the rate of preterm CS (0.9% to 1.6%; P < .001) increased.
3.3. Perinatal outcome
Figure 2 and Supporting Information Table S2 show trends in perinatal mortality. Overall perinatal mortality from 20 weeks of gestation decreased from 6.7 to 5.7 per 1000 births (P < .001). Importantly, of the 0.7‰ reduction in perinatal mortality from 28 weeks, 0.5‰ occurred after 37 weeks, indicating that only a small part of the reduction was achieved between 28 and 37 weeks.
FIGURE 2.
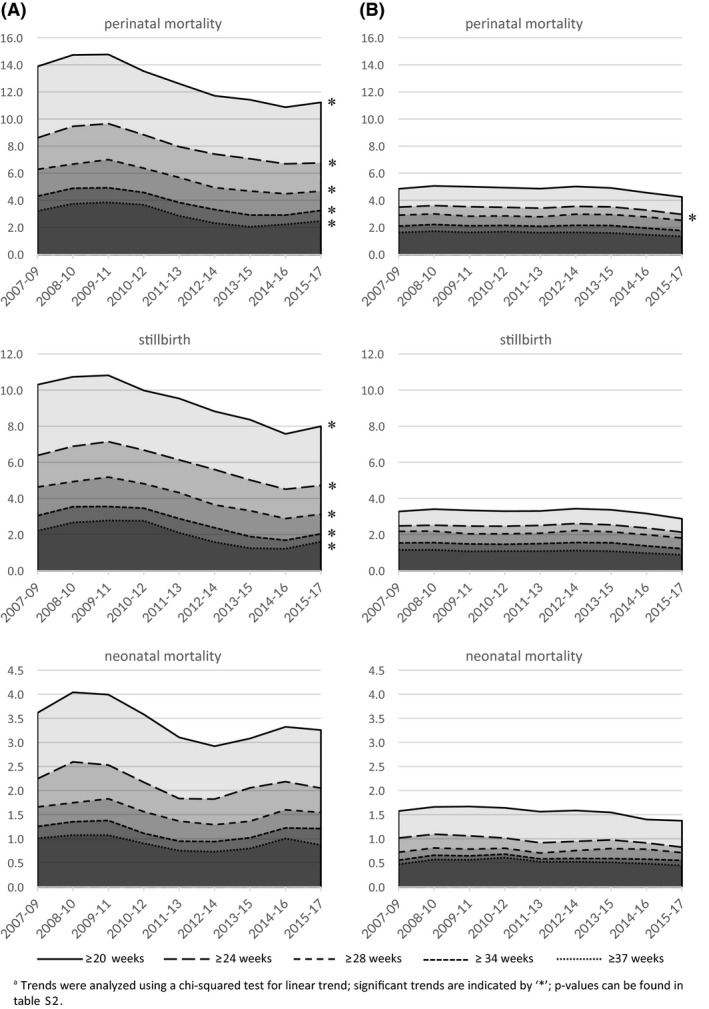
Stillbirth and neonatal and perinatal mortality from 20, 24 28, 34 and 37 weeks of gestation in (A) singleton pregnancies complicated by small for gestational age, hypertension, (pre)eclampsia or prelabor rupture of membranes and (B) singleton pregnancies not complicated by any of these conditions, per 1000 (live) births: Victoria, 2007‐2017a
In pregnancies complicated by SGA, hypertension, (pre)eclampsia or PROM, the perinatal mortality and stillbirth rate from 20 weeks of gestation decreased from 13 to 12 and 9.7 to 8.9 per 1000 births, respectively (P < .001). Comparable significant downward trends were seen in perinatal mortality and stillbirth rate from 24, 28, 34 and 37 weeks of gestation. The weekly prospective risk of stillbirth decreased strongly at 34+0‐36+6 (0.37 to 0.21 per 1000 births) and 37+0‐41+6 weeks (0.85 to 0.64 per 1000 births), whereas there was a more subtle decrease at 20+0‐27+6 (0.72 to 0.62 per 1000 births) and 28+0‐33+6 (0.23 to 0.16 per 1000 births; Figure 3). Lower neonatal mortality rates were observed in 2017 compared with 2007; however, this difference was not statistically significant (3.3 in 2007 vs 2.7 in 2017 per 1000 births from 20 weeks of gestation; P = .137).
FIGURE 3.

Average weekly prospective stillbirth risk at 20+0‐27+6, 28+0‐33+6, 34+0‐36+6 and 37+0‐41+6 weeks of gestation in (A) singleton pregnancies complicated by small for gestational age, hypertension, (pre)eclampsia or prelabor rupture of membranes and (B) singleton pregnancies not complicated by any of these conditions: Victoria, 2007‐2017
In pregnancies not complicated by SGA, hypertension, (pre)eclampsia or PROM, perinatal mortality from 24 weeks of gestation decreased significantly from 3.5 to 2.8 per 1000 births (P = .007). Observed perinatal mortality rates from 20, 28, 34 and 37 weeks of gestation were lower in 2017 compared with 2007, but the results were not statistically significant. Similarly, observed rates of stillbirth (3.1 in 2007 vs 2.7 in 2017 per 1000 births from 20 weeks of gestation; P = .063) and neonatal mortality (1.6 in 2007 vs 1.5 in 2017 per 1000 births from 20 weeks of gestation; P = .087) were lower in 2017 from any gestational age, but none of these results were statistically significant. For average weekly prospective stillbirth risk, a decrease was only seen beyond 37 weeks (Figure 3).
4. DISCUSSION
In Victoria there has been a significant increase in preterm birth from 5.9% in 2007 to 6.4% in 2017, which is driven by an increase in iatrogenic, late preterm birth. Comparable trends are seen for high‐risk pregnancies complicated by SGA and hypertension, and in pregnancies not complicated by SGA, hypertension, (pre)eclampsia or PROM. A decrease in prospective stillbirth risk and perinatal mortality was seen in pregnancies complicated by SGA, hypertension, (pre)eclampsia or PROM. In pregnancies not complicated by SGA, hypertension, (pre)eclampsia or PROM, our research showed no significant decrease in weekly prospective stillbirth risk before 37+0 weeks of gestation and perinatal mortality from 28 weeks of gestation.
Our study uses a large and recent data set from the validated Victorian Perinatal Data Collection.14 Nevertheless, routinely collected data have intrinsic limitations. Trends and associations can be adequately described, but causality can only be hypothesized, and residual confounding remains an important factor to consider. Despite adequate power, small changes and trends can remain unnoticed. Using diagnostic codes, our study could be subject to coding error and possible underreporting of certain conditions. No information on the use of assisted reproductive technology, socioeconomic status of the mother, neonatal morbidity and maternal outcomes was available for analyses.13
The increase in preterm and iatrogenic preterm birth in Victoria mirrors trends in other parts of Australia11 and overseas.6, 7, 8 In South Australia, preterm birth in singleton pregnancies increased from 5.1% to 7.1% between 1986 and 2014, driven by an increase in iatrogenic preterm birth (1.6% to 3.2%).11 In eight European countries comparable trends were seen (4.4%‐7.9% in 1996 to 5.3%‐8.7% in 2008).6 Contrary to our results however, in 11 other European countries a stable or decreasing preterm birth rate was noted, as was the case in the USA between 2006 and 2014 (12.8% to 9.6%).6, 9, 10 In six European countries non‐spontaneous preterm birth increased, and in Canada, Denmark and Finland the clinician‐initiated obstetric interventions among late preterm birth increased.6, 8 Previous studies reported declining perinatal mortality in all singletons, including in South Australia and Scotland.11, 18 Our study shows similar results for all singleton pregnancies and complicated singleton pregnancies, but no significant decrease in perinatal mortality from 28 weeks of gestation was noted in singleton pregnancies not complicated by SGA, hypertension, (pre)eclampsia or PROM.
Australian and international guidelines recommend IOL at 37 weeks of gestation for pregnancies complicated by preeclampsia, gestational hypertension with blood pressures below 160/110 mmHg, PROM without signs of infection or fetal compromise, and SGA diagnosed after 32 weeks of gestation.19, 20, 21 The substantial increase in iatrogenic preterm birth found in our study cannot be explained by these recommendations. However, as was argued previously for twin pregnancies,13 increased awareness and monitoring could have contributed to more interventions in the late preterm phase, and in pregnancies with minor or less well studied conditions.22 Increased societal risk adversity and improvements in neonatal care, and subsequent survival of the neonate and the perception of low morbidity could also have lowered the threshold at which timing of birth is considered.13, 23, 24, 25
Spontaneous preterm birth significantly decreased in our study period. This may be a reflection of advances in obstetric management in the prevention of spontaneous preterm birth, or of other influences with a less clear impact, including vaccination and lifestyle improvements.26, 27 Alternatively, it can be reasoned that it is a direct result of more iatrogenic preterm birth.13
Changes in the baseline characteristics in our study were small and their clinical relevance is doubtful. The number of women from Asian descent increased, but a recent meta‐analysis showed no significant association between Asian ethnicity and preterm birth risk.28 The effect of decreased smoking before 20 weeks and increased smoking after 20 weeks on the preterm birth rates remains speculative.
Our study showed a strong increase in iatrogenic preterm birth, not only in pregnancies complicated by SGA, hypertension, (pre)eclampsia or PROM, but also in pregnancies not complicated by any of these conditions. With the latter we intended to select a group of pregnancies with overall a fairly low perinatal mortality risk, compared with the higher overall perinatal mortality risk in pregnancies complicated by any of the complications mentioned before. In the high‐risk pregnancies complicated by SGA, hypertension, (pre)eclampsia or PROM, the increase in iatrogenic preterm birth was accompanied by a decrease in perinatal mortality, especially in stillbirth rate. Similar results were recently published for pregnancies with suspected SGA.29, 30 Although causality cannot be established, our study shows approximately 250 extra iatrogenic preterm births and 4 fewer stillbirths annually from 20 weeks of gestation between 2007 and 2017. In the relatively lower risk pregnancies, the increase in (iatrogenic) preterm birth was not accompanied by a significant decrease in stillbirth rate from 20 weeks (approximately 750 extra iatrogenic preterm births, three fewer stillbirths). Moreover, the prospective approach that we used to more accurately estimate the weekly stillbirth risks showed no decrease in preterm stillbirth risk in relatively low‐risk pregnancies. Although the overall perinatal mortality from 24 weeks of gestation in these pregnancies did decrease significantly, no significant decrease was seen from 28 to 37 weeks of gestation, which is the group where iatrogenic preterm birth is mostly increasing. This calls for an ongoing discussion among professionals, as we argued previously for twin pregnancies, on whether in singleton pregnancies not complicated by SGA, hypertension, (pre)eclampsia or PROM with a relatively low perinatal mortality risk, the effort to reduce stillbirth by more and earlier iatrogenic preterm birth is being effective, and what costs, both in neonatal morbidity and in monetary cost, society is prepared to pay.13 As awareness on the negative influence of (late) preterm birth on neurocognitive development increases, the long‐term benefits of prolonging pregnancy should not be overlooked and should be taken into the complex consideration of timing of birth.3, 13
5. CONCLUSION
The preterm birth rate in singleton pregnancies in Victoria is increasing, driven by an increase in iatrogenic preterm birth. This occurred both in pregnancies complicated by hypertension or SGA, and in pregnancies not complicated by SGA, hypertension, (pre)eclampsia or PROM. While perinatal mortality decreased in pregnancies complicated by SGA, hypertension, (pre)eclampsia or PROM, no significant reduction in perinatal mortality from 28 weeks of gestation or the preterm weekly prospective stillbirth risk was noted in pregnancies not complicated by any of these conditions. These results stress the need for an ongoing debate and research on optimal timing of delivery, including in singleton pregnancies not complicated by SGA, hypertension, (pre)eclampsia or PROM.
CONFLICT OF INTEREST
BWM reports consultancy for ObsEva, Merck and Guerbet. MAD is a part‐time employee of the Consultative Councils Unit, which manages the Victorian Perinatal Data Collection. EMW is the CEO of Safer Care Victoria. All other authors report no conflict of interest.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to the Consultative Council on Obstetric and Paediatric Mortality and Morbidity (CCOPMM) for providing access to the data used for this project and for the assistance of the staff at Safer Care Victoria. The conclusions, findings, opinions and views or recommendations expressed in this paper are strictly those of the author(s). They do not necessarily reflect those of CCOPMM.
Burger RJ, Temmink JD, Wertaschnigg D, et al. Trends in singleton preterm birth in Victoria, 2007 to 2017: A consecutive cross‐sectional study. Acta Obstet Gynecol Scand. 2021;100:1230–1238. 10.1111/aogs.14074
Funding information
BWM is supported by an NHMRC Investigator Grant (GNT1176436). EMW is supported by an NHMRC Program Grant (APP1113902) and a Partnership Grant (APP1151853). DW is supported by a grant from the Paracelsus Medical Private University (PMU Research Fund – PMU FFF Number: L‐18/02/006‐WET). The author(s) received no financial support for the research, authorship and/or publication of this article.
REFERENCES
- 1.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162‐2172. [DOI] [PubMed] [Google Scholar]
- 2.Woythaler MA, McCormick MC, Smith VC. Late preterm infants have worse 24‐month neurodevelopmental outcomes than term infants. Pediatrics. 2011;127:e622‐e629. [DOI] [PubMed] [Google Scholar]
- 3.Zwertbroek EF, Franssen MTM, Broekhuijsen K, et al. Neonatal developmental and behavioral outcomes of immediate delivery versus expectant monitoring in mild hypertensive disorders of pregnancy: 2‐year outcomes of the HYPITAT‐II trial. Am J Obstet Gynecol. 2019;221:154.e1‐154.e11. [DOI] [PubMed] [Google Scholar]
- 4.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261‐269. [DOI] [PubMed] [Google Scholar]
- 5.Kugelman A, Colin AA. Late preterm infants: near term but still in a critical developmental time period. Pediatrics. 2013;132:741‐751. [DOI] [PubMed] [Google Scholar]
- 6.Zeitlin J, Szamotulska K, Drewniak N, et al. Preterm birth time trends in Europe: a study of 19 countries. BJOG. 2013;120:1356‐1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chawanpaiboon S, Vogel JP, Moller A‐B, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7:e37‐e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richards JL, Kramer MS, Deb‐Rinker P, et al. Temporal Trends in Late Preterm and Early Term Birth Rates in 6 High‐Income Countries in North America and Europe and Association With Clinician‐Initiated Obstetric Interventions. JAMA. 2016;316:410‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purisch SE, Gyamfi‐Bannerman C. Epidemiology of preterm birth. Semin Perinatol. 2017;41:387‐391. [DOI] [PubMed] [Google Scholar]
- 10.van Zijl MD, Koullali B, Oudijk MA, et al. Trends in preterm birth in singleton and multiple gestations in the Netherlands 2008–2015: a population‐based study. Eur J Obstet Gynecol Reprod Biol. 2020;247:111‐115. [DOI] [PubMed] [Google Scholar]
- 11.Verburg PE, Dekker GA, Venugopal K, et al. Long‐term Trends in Singleton Preterm Birth in South Australia From 1986 to 2014. Obstet Gynecol. 2018;131:79‐89. [DOI] [PubMed] [Google Scholar]
- 12.Consultative Council on Obstetric and Paediatric Mortality and Morbidity (CCOPMM) . Victoria's Mothers, Babies and Children report 2017 Melbourne: CCOPMM; 2019.
- 13.Burger RJ, Temmink S, Wertaschnigg D, et al. Trends in preterm birth in twin pregnancies in Victoria, Australia, 2007–2017. Aust N Z J Obstet Gynaecol. 2020; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 14.Flood MM, McDonald SJ, Pollock WE, Davey MA. Data accuracy in the Victorian Perinatal Data Collection: Results of a validation study of 2011 data. Health Inf Manag. 2017;46:113‐126. [DOI] [PubMed] [Google Scholar]
- 15.Dobbins TA, Sullivan EA, Roberts CL, Simpson JM. Australian national birthweight percentiles by sex and gestational age, 1998–2007. Med J Aust. 2012;197:291‐294. [DOI] [PubMed] [Google Scholar]
- 16.Davies‐Tuck ML, Davey MA, Wallace EM. Maternal region of birth and stillbirth in Victoria, Australia 2000–2011: a retrospective cohort study of Victorian perinatal data. PLoS One. 2017;12:e0178727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joseph KS. The fetuses‐at‐risk approach: clarification of semantic and conceptual misapprehension. BMC Pregnancy Childbirth. 2008;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norman JE, Morris C, Chalmers J. The effect of changing patterns of obstetric care in Scotland (1980–2004) on rates of preterm birth and its neonatal consequences: perinatal database study. PLoS Med. 2009;6:e1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institute for Health and Care Excellence (NICE) . Hypertension in pregnancy: diagnosis and management (NICE guidelines 133). 2019. [PubMed]
- 20.Morris JM, Roberts CL, Bowen JR, et al. Immediate delivery compared with expectant management after preterm pre‐labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444‐452. [DOI] [PubMed] [Google Scholar]
- 21.Royal College of Obstetricians & Gynaecologists . Small‐for‐Gestational‐Age Fetus, Investigation and Management (Green‐top Guideline No. 31) London: RCOG;. 2013, updated 2014.
- 22.Sovio U, White IR, Dacey A, Pasupathy D, Smith GCS. Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the Pregnancy Outcome Prediction (POP) study: a prospective cohort study. Lancet. 2015;386:2089‐2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shepherd E, Salam RA, Middleton P, et al. Antenatal and intrapartum interventions for preventing cerebral palsy: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev. 2017;8:CD012077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017;3:CD004454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coxon KRRBMP, Homer CMCP, Bisits AM, Sandall JRRHBMP, Bick DRBMP. Reconceptualising risk in childbirth. Midwifery. 2016;38:1‐5. [DOI] [PubMed] [Google Scholar]
- 26.Berghella V, Ciardulli A, Rust OA, et al. Cerclage for sonographic short cervix in singleton gestations without prior spontaneous preterm birth: systematic review and meta‐analysis of randomized controlled trials using individual patient‐level data. Ultrasound Obstet Gynecol. 2017;50:569‐577. [DOI] [PubMed] [Google Scholar]
- 27.Dodd JM, Jones L, Flenady V, Cincotta R, Crowther CA. Prenatal administration of progesterone for preventing preterm birth in women considered to be at risk of preterm birth. Cochrane Database Syst Rev. 2013;7:CD004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaaf JM, Liem SM, Mol BW, Abu‐Hanna A, Ravelli AC. Ethnic and racial disparities in the risk of preterm birth: a systematic review and meta‐analysis. Am J Perinatol. 2013;30:433‐450. [DOI] [PubMed] [Google Scholar]
- 29.Selvaratnam RJ, Davey MA, Anil S, McDonald SJ, Farrell T, Wallace EM. Does public reporting of the detection of fetal growth restriction improve clinical outcomes: a retrospective cohort study. BJOG. 2020;127:581‐589. [DOI] [PubMed] [Google Scholar]
- 30.Selvaratnam RJ, Davey MA, Mol BW, Wallace EM. Increasing obstetric intervention for fetal growth restriction is shifting birthweight centiles: a retrospective cohort study. BJOG. 2020;127:1074‐1080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


