Fig. 2.
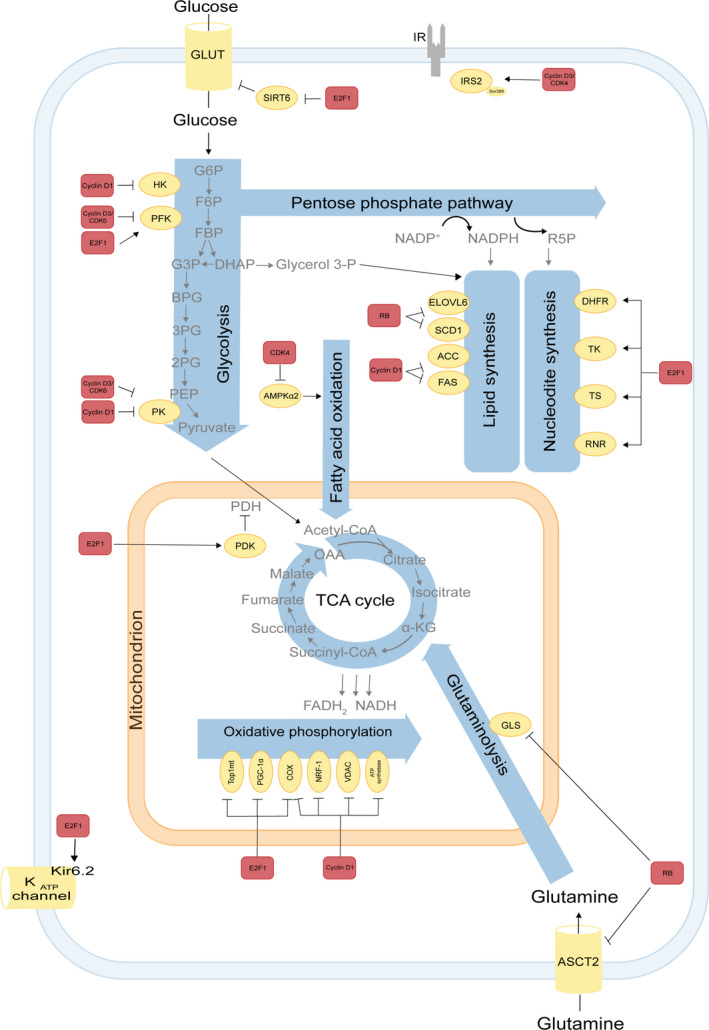
Participation of cell cycle regulators in metabolic pathways. The E2F‐pRB pathway is involved in several metabolic pathways. In glucose metabolism of pancreatic β‐cells E2F1 regulates Kir6.2, a key component of the KATP channel, controlling glucose‐induced insulin secretion [13]. Moreover, E2F1 also enhances glycolysis in bladder and prostate cancer cell lines through the SIRT6 that inhibits the transcription of several key glycolytic genes such as GLUT1 [22]. Furthermore, E2F1 stimulates glycolysis by upregulating the expression of the enzyme 6‐phophofructo‐2 kinase/fructose‐2,6‐bisphosphatase [20, 21]. E2F1 also induces expression of PDK1/4 in pancreatic cancer cells and PDK4 in the heart. PDKs inhibit PDH and thereby oxidative metabolism [23, 24]. In addition, associated to pRB E2F1 modulates the expression of key genes implicated in mitochondrial biogenesis or oxidative phosphorylation, such as mitochondrial topoisomerase 1 (Top1mt) [159], PGC‐1α [160], cytochrome c oxidase subunit 4 COX4, and cytochrome c oxidase subunit 7C [161, 162]. In nucleotide metabolism, E2F1 induces dihydrofolate reductase, thymidine kinase, TS and RNR [36, 38, 39]. Deletions of RB family members increase glutamine uptake and conversion to glutamate, mediated through elevated expression of the glutamine transporter ASCT2 and GLS1 activity in MEF [34] and upregulate enzymes involved in elongation, long chain fatty acid family member 6, and desaturation of fatty acids by stearoyl‐CoA desaturase 1 [163]. Cyclin D1 has an E2F1‐independent role in the control of oxidative metabolism. It directly modulates the activity of the transcription factor NRF1, the mitochondrial voltage‐dependent anion channel [122, 164], mitochondrial electron transport chain components COX and ATP synthase [165] thereby inhibiting oxidative phosphorylation. In addition, cyclin D1 inhibits HKII and pyruvate kinase (PK), which are involved in the glycolysis pathway as well as the lipogenic enzymes acetyl‐CoA carboxylase and fatty acid synthase levels [26]. In adipocytes, cyclin D3‐CDK4 complex phosphorylates IRS2 at serine 388, thereby creating a positive feedback loop that maintains adipocyte insulin signaling [17]. In addition, the CDK4 also represses FAO in an E2F1‐independent manner through direct phosphorylation and inhibition of AMPKα2. Cyclin D3–CDK6 kinase phosphorylates and inhibits the catalytic activity of two key enzymes in the glycolytic pathway, 6‐phosphofructokinase (isoform PFKP) and pyruvate kinase (isoform PKM2) in T‐cell acute lymphoblastic leukemia [27]. Interactions between the cell cycle machinery and metabolic enzymes indicated by arrows may be either direct or indirect. Color codes represent proteins belonging to the cell cycle machinery (red) or to metabolic pathways (yellow).
