FIGURE 2.
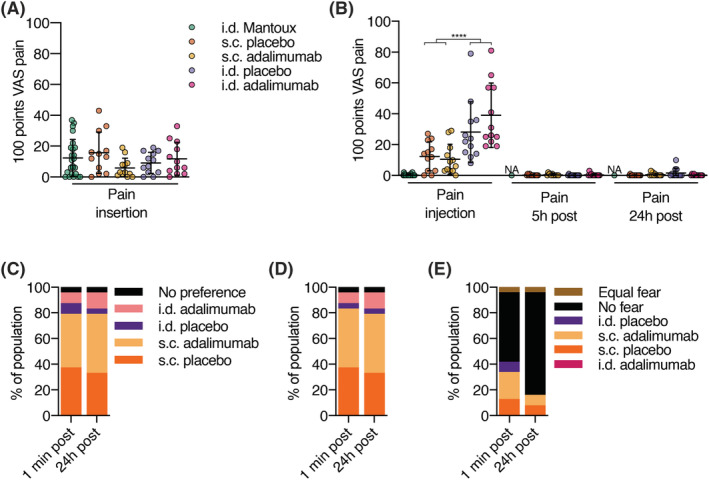
Volunteer reported outcomes indicate preference for subcutaneous (s.c.) administration vs. intradermal (i.d.) administration. Healthy volunteers were injected with a single dose of adalimumab in the upper thigh and placebo in the contralateral upper thigh administered i.d. using a hollow microneedle or s.c. using a conventional needle. Insertion and injection pain were normalized to the pain score during a Mantoux which the volunteers received during screening. (A) Visual analogue scale (VAS) pain scores for insertion pain. No differences were observed between s.c. and i.d. insertion pain (P = .68). (B) VAS pain scores for injection and postinjection pain. Injection pain was significantly (P < .0001) higher for i.d. compared to s.c. injection. Postinjection pain was not present. After injection, subjects were asked multiple choice questions about their preference, for (C) how they experienced the injection, (D) how they would like to get a hypothetical future injection, (E) and for which injection they had fear. (A‐E): n = 12 per group, except for Mantoux where n = 24. (A‐B): mean ± standard deviation; repeated‐measures ANOVA; **** P < .0001. NA: not available because not measured
