FIGURE 3.
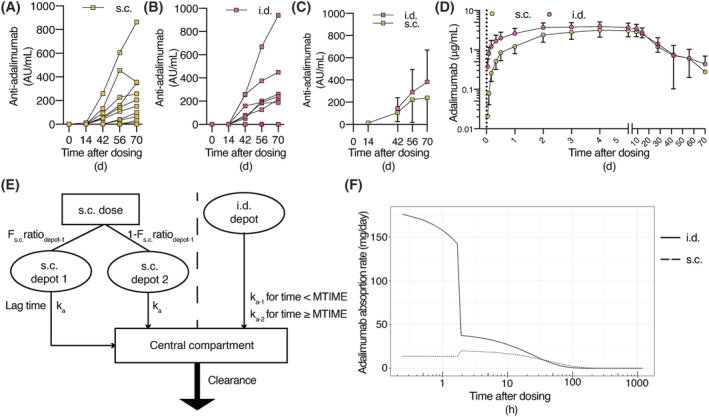
Pharmacokinetics of adalimumab and anti‐adalimumab antibodies after subcutaneous (s.c.) or intradermal (i.d.) injection. Mean anti‐adalimumab levels after (A) s.c. and (B) i.d. administration (n = 12 per administration type). (C) Average anti‐adalimumab levels for subjects with anti‐adalimumab antibodies (n = 10 for s.c. administration and n = 6 for i.d. administration). (D) Serum adalimumab concentrations over time (D, n = 10 for s.c. administration and n = 9 for i.d. administration, noncompartmental analysis of subjects without leakage during injection). (C‐D) Mean ± standard deviation. (E) Schematic depiction of population PK model. (F) Adalimumab absorption kinetics over time after adalimumab administration following microneedle (i.d.) or s.c. administration (typical population PK model). F: relative bioavailability; ka: absorption rate constant
