Abstract
Objective
To determine the relationship between 25-hydroxyvitamin D (25[OH]D) values and subsequent cancer incidence and mortality.
Patients and Methods
We identified all adult patients living in Olmsted County, Minnesota between 01/01/2005 and 12/31/2011 who had at least one 25(OH)D measurement and no prior diagnosis of cancer. Cancer outcomes were retrieved starting 30 days after 25(OH)D measurement and until patients’ final clinical visit as an Olmsted County resident, 12/31/2014, or death. Cox proportional hazards regression was used to analyze data.
Results
A total of 8,700 individuals had a 25(OH)D measurement and no prior history of cancer, with a mean 25(OH)D value of 29.7±12.8 ng/mL. The mean age was 51.5±16.4 years, and most were women (78.1%) and white (85.7%). A total of 761 individuals developed cancer (skin cancer, n=360; non-skin cancer, n=401) over a median (interquartile range) follow-up duration of 4.6 (3.4–6.1) years. Compared with subjects with 25(OH)D values 20–50 ng/mL (reference group), those with 25(OH)D values <12 ng/mL had a greater non-skin cancer incidence (hazard ratio [HR], 1.56; 95% CI, 1.03–2.36; P=.04) after adjustment. There was no association between 25(OH)D values and total cancer or skin cancer incidence. Compared with subjects from the reference group, 25(OH)D <12 ng/mL (HR, 2.35; 95% CI, 1.01–5.48; P=.047) and 12–19 ng/mL (HR, 2.10; 95% CI, 1.05–4.22; P=.04) were associated with increased cancer mortality.
Conclusion
Low 25(OH)D levels were associated with increased risk of incident non-skin cancer and cancer-related mortality.
Keywords: vitamin D, epidemiology, nutrition, population, malignancy, neoplasm
1. Introduction
Low vitamin D status is widely prevalent and has been associated with increased cancer incidence and mortality in multiple observational studies.1–9 Animal models and in vitro studies suggest that vitamin D affects cancer biology through various mechanisms — cell differentiation, apoptosis, angiogenesis, cancer cell differentiation, inflammation, and immune modulation.10 Specific vitamin D receptor gene polymorphisms leading to loss or gain of function modify the risk of multiple cancer subtypes.11–15 Recent meta-analyses of randomized controlled trials (RCTs) evaluating vitamin D supplementation demonstrated 13–16% decreased risk in cancer mortality compared with placebo; however, the studies failed to demonstrate a significant effect on cancer incidence.16, 17 In the VITAL study, one of the largest of such RCTs, vitamin D supplementation decreased the risk of cancer-related death (hazard ratio [HR] 0.75, 95% CI 0.59–0.96) in a subset of patients receiving at least 2 years of supplementation (2000 IU per day).18 However, there was no significant effect on cancer incidence.
Several authors have proposed that the duration of vitamin D exposure and study follow up in most RCTs have been insufficient to affect cancer incidence, potentially explaining the conflicting observations of cancer incidence in observational versus interventional studies.16 RCTs have also been limited by significant proportions of the control arms often consuming vitamin D supplements.17
Similar to all-cause mortality, the risk of cancer-related death may have an inverse J-shaped association, with greatest risk at 25-hydroxyvitamin D [25(OH)D] levels <12 ng/mL (to convert to nmol/L, multiply by 2.496).9 Moreover, we previously reported differences in risk by ethnicity, with only whites having greater all-cause mortality risk with low serum 25(OH)D concentrations.9 Recent studies suggest that specific cancer sites may be differentially affected by 25(OH)D status.19, 20 Colorectal,20 breast,21 prostate,6 and ovarian22 cancers have been linked to low serum 25(OH)D levels in large cohort studies. Pancreatic cancer incidence has been linked to high serum 25(OH)D levels in a large case-control study.23
Most previous trials and observational studies of vitamin D and cancer incidence and mortality have been limited by small cohorts, low ethnic diversity, and sparse analyses of cancer subtypes, as well as limited vitamin D exposure time in RCTs. Our objective was to perform a population-based retrospective cohort study in the upper Midwest region of the United States of the relationship between vitamin D status and risk of cancer by subtype and cancer-related death. We hypothesized that we would observe an increased cancer incidence and cancer mortality in persons with low baseline 25(OH)D status.
2. Materials and Methods
2.1. Rochester Epidemiology Project and setting
In an extension of our previous work and methodology,9, 24 we conducted a population-based retrospective cohort study using the Rochester Epidemiology Project (REP). The REP is a medical records-linkage infrastructure, allowing for population-based medical research in Olmsted County, Minnesota.25, 26 The major health systems in Olmsted County include Mayo Clinic and Olmsted Medical Center, providing a range of primary care through quaternary-level services.25 The REP provides more than 50 years of data for over 500,000 unique individuals and 6.2 million person-years of follow-up, capturing 95% of the county’s population.25, 26
Olmsted County, Minnesota is located at 44°N latitude in the Midwestern United States. There were 144,248 people residing in Olmsted County, MN in 2010.27 In comparison to the U.S. population in 2010, Olmsted County had less ethnic diversity (86% versus 72% white), was more educated (94% versus 85% high school graduates) and was wealthier ($64,090 versus $51,914 median household income).26 However, the population resembled that of the overall Upper Midwest.27
2.2. Patient selection and outcomes
We identified all adults residing in Olmsted County between January 1, 2005 and December 31, 2011 who had at least one serum 25(OH)D measurement. The initial measurement was considered the index measurement. Cancer outcomes were based on diagnosis codes retrieved starting 30 days after 25(OH)D measurement. All malignant neoplasms were included and identified using International Classification of Diseases, Ninth Revision (ICD-9) codes 140–208.92 and 230–234.9 (Supplemental Table). Patients were followed until their last clinical visit as an Olmsted County resident; until December 31, 2014; or until death, whichever came first.
We collected data regarding each patient’s age, sex, body mass index (BMI), race, time of year, Charlson Comorbidity Index (CCI), socioeconomic status, hypertension, and smoking history, all of which were obtained at the time of index 25(OH)D measurement. Socioeconomic status was determined by the HOUsing-based index of SocioEconomic Status (HOUSES) index derived from real property data.28 Four standardized scores (each score with mean of 0 and standard deviation [SD] of 1) are summated for each subject’s residence: Assessed housing value, Area of living space, Number of bedrooms, and Number of bathrooms. Patient addresses in the medical record were directly linked to publicly available property data. HOUSES index corresponded with the time of index 25(OH)D measurement.
The study was approved by the Mayo Clinic Institutional Review Board, and research authorization was granted for retrospective chart review of all included records.
2.3. Laboratory methods
All 25(OH)D measurements were performed at Mayo Medical Laboratories during the study interval by isotope-dilution liquid chromatography-tandem mass spectrometry. For this study, 25(OH)D refers to the sum of 25(OH)D2 and 25(OH)D3. Interassay coefficients of variation for 25(OH)D2 were 6–14% and for 25(OH)D3 were 6–13% in control samples. The recovery of analyte spiked into patient samples was 82–115% (mean, 102%) of predicted for 25(OH)D2 and 88–115% (mean, 103%) for 25(OH)D3. The limit of detection was 4 ng/mL. Throughout the study interval, Mayo Clinic Laboratory participated in the CDC Vitamin D standardization program (Ravinder Singh PhD, written communication, August 22, 2014).
2.4. Statistical analyses
All analyses were performed with SAS, version 9.4 (SAS Institute, Cary, NC) and R version 3.6.2 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria). Serum 25(OH)D was examined as a categorical variable using predetermined ranges of interest: 25(OH)D values <12, 12–19, 20–50 (reference category), and >50 ng/mL. The Kaplan-Meier method was used to calculate the probability of cancer and the probability of surviving without cancer for each of the four categories of 25(OH)D values. Cox proportional hazards regression was used to assess the relationship between 25(OH)D values and risk of cancer. Multivariable analysis was adjusted for age, sex, race, month of 25(OH)D measurement, BMI, CCI, HOUSES Z score, smoking history, hypertension, and osteoporosis/osteopenia. The functional form of continuous variables was assessed using Martingale residuals and the proportional hazards assumption was assessed with plots of the Schoenfeld residuals. After looking at survival free of any cancer, we performed similar analyses looking at the most common non-skin cancer subtypes: colorectal, breast, and lung. We performed a sensitivity analysis limited to the subset of patients with at least one year of follow-up after 25(OH)D measurement to exclude all cancers that were diagnosed within 1 year of baseline 25(OH)D measurement.
Among patients who were diagnosed with a cancer during follow-up, we analyzed time from cancer diagnosis to death. The Kaplan-Meier method was used to calculate the probability of surviving after cancer for each of the four categories of 25(OH)D values. Cox proportional hazards regression was used to assess the relationship between 25(OH)D values and risk of cancer-related death. Multivariable analysis was adjusted for race, CCI, and smoking history.
3. Results
Between January 1, 2005 and December 31, 2011, a total of 11,002 adults in Olmsted County had a 25(OH)D measurement. From this group, 10 adults were excluded due to lack of research authorization, 390 were excluded due to cancer diagnosis within 30 days subsequent to 25(OH)D level, and 1,902 were excluded due to cancer diagnosis prior to 25(OH)D measurement. The final study group included 8,700 adults residing in Olmsted County with baseline 25(OH)D evaluation and no prior history of cancer (Table 1). The mean (SD) age of the study population was 51.5 (16.4) years, and 78.1% were women. White race was reported in 85.7% of study subjects. The mean (SD) 25(OH)D value among the study population was 29.7 (12.8) ng/mL. A total of 761 individuals developed cancer (skin cancer, n=360; non-skin cancer, n=401) over a median (interquartile range) follow-up duration of 4.6 (3.4–6.1) years.
Table 1.
Baseline characteristics of Olmsted County residents with a serum 25(OH)D measurement between 1/1/2005 and 12/31/2011 and no prior history of cancer.
| Serum 25(OH)D categories (ng/mL) | ||||||
|---|---|---|---|---|---|---|
| Characteristics | Total | <12 | 12–19 | 20–50 | >50 | P value |
| n | 8700 | 515 | 1331 | 6420 | 434 | |
| 25(OH)D, Mean (SD), ng/mL | 29.7 (12.8) | 8.5 (2.1) | 15.9 (2.3) | 32.1 (7.6) | 61.2 (15.1) | |
| Age, mean (SD), years | 51.5 (16.4) | 47.8 (16.3) | 49.5 (16.0) | 52.1 (16.4) | 52.7 (17.6) | .10a |
| Age groups, n (%) | ||||||
| 18–49 yr | 3848 (44.2) | 285 (55.3) | 658 (49.4) | 2727 (42.5) | 178 (41.0) | |
| 50–64 yr | 3066 (35.2) | 162 (31.5) | 459 (34.5) | 2291 (35.7) | 154 (35.5) | |
| ≥65 yr | 1786 (20.5) | 68 (13.2) | 214 (16.1) | 1402 (21.8) | 102 (23.5) | |
| Sex, n (%) | <.001 | |||||
| Women | 6796 (78.1) | 376 (73.0) | 984 (73.9) | 5045 (78.6) | 391 (90.1) | |
| Race, n (%) | <.001 | |||||
| Non-white | 1240 (14.3) | 207 (40.2) | 370 (27.8) | 646 (10.1) | 17 (3.9) | |
| Season, n (%) | <.001 | |||||
| Winter, Dec-Feb | 2176 (25.0) | 177 (34.4) | 400 (30.1) | 1521 (23.7) | 78 (18.0) | |
| Spring, Mar-May | 2267 (26.1) | 166 (32.2) | 425 (31.9) | 1556 (24.2) | 120 (27.6) | |
| Summer, Jun-Aug | 1969 (22.6) | 78 (15.1) | 195 (14.7) | 1572 (24.5) | 124 (28.6) | |
| Fall, Sept-Nov | 2288 (26.3) | 94 (18.3) | 311 (23.4) | 1771 (27.6) | 112 (25.8) | |
| Charlson Comorbidity Index, mean (SD) | 2.5 (2.5) | 2.6 (2.9) | 2.5 (2.7) | 2.4 (2.4) | 2.6 (2.4) | .04a |
| BMI, mean (SD) | 29.0 (7.6) | 32.2 (10.1) | 31.2 (8.3) | 28.5 (7.1) | 25.5 (6.1) | −.22b |
| HOUSES Z, c mean (SD) | −0.10 (3.51) | −1.51 (3.16) | −0.96 (3.39) | 0.16 (3.50) | 0.40 (3.58) | .12b |
| Ever Smoked d | 3841 (47.9) | 261 (55.4) | 641 (52.5) | 2750 (46.5) | 189 (46.0) | <.001 |
| Hypertension | 3294 (37.9) | 221 (42.9) | 528 (39.7) | 2389 (37.2) | 156 (35.9) | .03 |
| Hyperlipidemia | 4291 (49.3) | 214 (41.6) | 636 (47.8) | 3222 (50.2) | 219 (50.5) | .001 |
| Osteopenia/Osteoporosis | 2998 (34.5) | 130 (25.3) | 376 (28.2) | 2298 (35.8) | 194 (44.9) | <.001 |
Spearman Correlation
Pearson Correlation
Available in 8206
Available in 8012
In the univariate analysis, older age, male sex, CCI, smoking history, and hypertension were individually associated with subsequent development of any cancer (Table 2). Non-white race was inversely associated with development of any cancer. Compared with the reference category, 25(OH)D 12–19 ng/mL (HR 0.72; 95% CI, 0.58 – 0.90), but not <12 ng/mL or >50 ng/mL, was associated with decreased risk of development of any cancer (Table 2; Supplemental Figure).
Table 2.
Associations between serum 25(OH)D values and any cancer. Univariate and multivariable hazard ratios are from Cox proportional hazards modeling of time to any cancer and specific cancer subgroups.a,b
| Risk Factor | Univariate HR (95% CI) | Multivariable HR (95% CI)* | |
|---|---|---|---|
| Total Cancer | Age (per decade) | 1.50 (1.43, 1.58) | 1.41 (1.31, 1.53) |
| Male Sex | 1.32 (1.12, 1.55) | 1.41 (1.18, 1.69) | |
| Non-white Race | 0.49 (0.37, 0.65) | 0.60 (0.44, 0.82) | |
| Charlson Comorbidity Index (per unit) | 1.17 (1.14, 1.20) | 1.03 (0.99, 1.07) | |
| BMI (per kg/m2) | 0.99 (0.98, 1.00) | 1.00 (0.99, 1.01) | |
| HOUSES Z (per unit) | 0.98 (0.96, 1.00) | 1.00 (0.98, 1.03) | |
| Ever Smoked (Yes vs No) | 1.48 (1.27, 1.71) | 1.33 (1.13, 1.55) | |
| Hypertension (Yes vs No) | 1.82 (1.58, 2.10) | 1.02 (0.85, 1.22) | |
| Osteoporosis/Osteopenia (Yes vs No) | 1.84 (1.59, 2.12) | 1.11 (0.93, 1.33) | |
| Vitamin D | |||
| 25(OH)D 20–50 ng/mL | 1.0 | 1.0 | |
| 25(OH)D <12 ng/mL | 0.88 (0.63, 1.21) | 1.17 (0.82, 1.66) | |
| 25(OH)D 12–19 ng/mL | 0.72 (0.58, 0.90) | 0.85 (0.67, 1.08) | |
| 25(OH)D >50 ng/mL | 1.32 (0.98, 1.78) | 1.32 (0.96, 1.80) | |
| Skin Cancer | Age (per decade) | 1.66 (1.55, 1.78) | 1.62 (1.44, 1.82) |
| Male Sex | 1.41 (1.12, 1.77) | 1.72 (1.33, 2.23) | |
| Non-white Race | 0.21 (0.12, 0.39) | 0.31 (0.16, 0.58) | |
| Charlson Comorbidity Index (per unit) | 1.18 (1.14, 1.22) | 0.98 (0.93, 1.04) | |
| BMI (per kg/m2) | 0.98 (0.96, 0.99) | 0.99 (0.97, 1.01) | |
| HOUSES Z (per unit) | 1.01 (0.98, 1.04) | 1.02 (0.99, 1.06) | |
| Ever Smoked (Yes vs No) | 1.27 (1.02, 1.57) | 1.12 (0.89, 1.40) | |
| Hypertension (Yes vs No) | 1.93 (1.57, 2.38) | 1.08 (0.83, 1.39) | |
| Osteoporosis/Osteopenia (Yes vs No) | 2.44 (1.98, 3.01) | 1.35 (1.04, 1.74) | |
| Vitamin D | |||
| 25(OH)D 20–50 ng/mL | 1.0 | 1.0 | |
| 25(OH)D <12 ng/mL | 0.35 (0.18, 0.71) | 0.63 (0.31, 1.29) | |
| 25(OH)D 12–19 ng/mL | 0.49 (0.34, 0.71) | 0.68 (0.46, 1.01) | |
| 25(OH)D >50 ng/mL | 1.28 (0.84, 1.95) | 1.15 (0.73, 1.81) | |
| Non-Skin Cancer | Age (per decade) | 1.38 (1.29, 1.47) | 1.27 (1.15, 1.41) |
| Male Sex | 1.24 (0.99, 1.55) | 1.19 (0.93, 1.53) | |
| Non-white Race | 0.75 (0.54, 1.04) | 0.84 (0.58, 1.20) | |
| Charlson Comorbidity Index (per unit) | 1.16 (1.13, 1.20) | 1.06 (1.01, 1.12) | |
| BMI (per kg/m2) | 1.00 (0.99, 1.02) | 1.00 (0.99, 1.02) | |
| HOUSES Z (per unit) | 0.96 (0.93, 0.99) | 0.99 (0.96, 1.02) | |
| Ever Smoked (Yes vs No) | 1.69 (1.38, 2.08) | 1.56 (1.25, 1.95) | |
| Hypertension (Yes vs No) | 1.73 (1.42, 2.10) | 0.97 (0.75, 1.24) | |
| Osteoporosis/Osteopenia (Yes vs No) | 1.43 (1.17, 1.74) | 0.93 (0.72, 1.18) | |
| Vitamin D | |||
| 25(OH)D 20–50 ng/mL | 1.0 | 1.0 | |
| 25(OH)D <12 ng/mL | 1.42 (0.98, 2.05) | 1.56 (1.03, 2.36) | |
| 25(OH)D 12–19 ng/mL | 0.96 (0.72, 1.28) | 1.01 (0.74, 1.37) | |
| 25(OH)D >50 ng/mL | 1.37 (0.90, 2.08) | 1.50 (0.97, 2.30) |
The multivariable model was also adjusted for season
Time 0 is 30 days after medical visit
In the multivariable analysis, older age (per decade, HR 1.41; 95% CI, 1.31–1.53), male sex (HR 1.41; 95% CI, 1.18–1.69), and history of smoking (HR 1.33; 95% CI, 1.13–1.55) were associated with development of any cancer (Table 2). Non-white race was associated with lower risk of cancer development (HR 0.60; 95% CI, 0.44–0.82). There was no interaction between race and low 25(OH)D on the incidence of cancer. There were no significant associations between levels of 25(OH)D with development of any cancer (Table 2; Figure 1).
Figure 1.
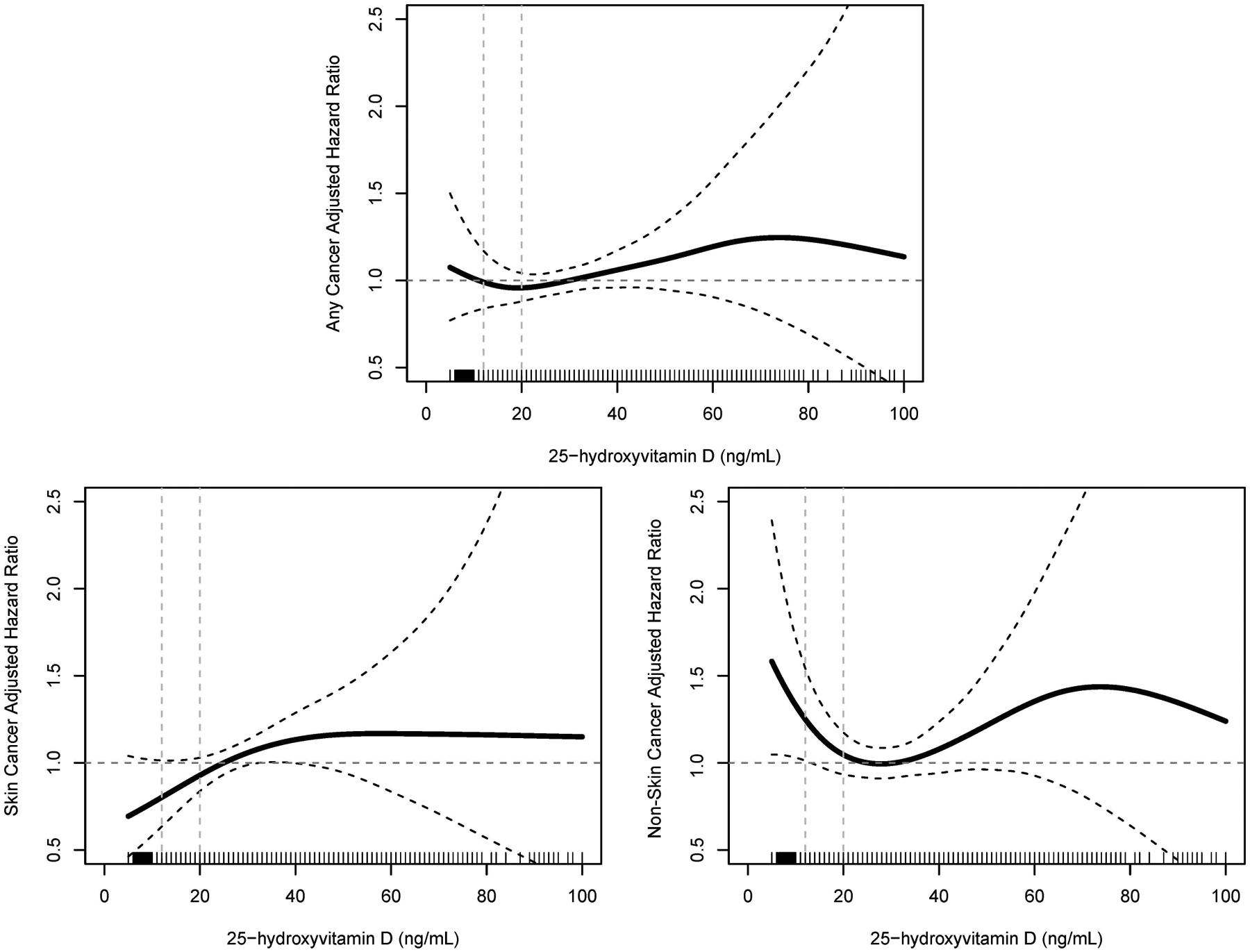
Spline plots displaying hazard ratios for (A) any cancer, (B) skin cancer, and (C) non-skin cancer, with serum 25(OH)D values as a continuous variable. SI conversion factors: To convert 25(OH)D values to mmol/L, multiply by 2.496. 25(OH)D, 25-hydroxyvitamin D.
We examined the outcomes of skin cancer and non-skin cancer separately. In the univariate analysis of development of skin cancer, older age, male sex, CCI, BMI, smoking history, and hypertension were associated with development of skin cancer, and non-white race was inversely associated with development of skin cancer (Table 2). Compared with the reference group, 25(OH)D <12 ng/mL (HR 0.35; 95% CI, 0.18–0.71) and 12–19 ng/mL (HR 0.49; 95% CI, 0.34–0.71), but not >50 ng/mL, were associated with decreased risk of development of skin cancer. In the multivariable analysis, older age (per decade, HR 1.62; 95% CI, 1.44–1.82) and male sex (HR 1.72; 95% CI, 1.33–2.23) were significantly associated with development of skin cancer, and non-white race (HR 0.31; 95% CI, 0.16–0.58) was inversely associated with development of skin cancer. 25(OH)D was not associated with development of skin cancer (Table 2; Figure 1).
For non-skin cancers, on univariate analysis, older age, CCI, HOUSES Z score, smoking history, and hypertension were associated with development of non-skin cancer (Table 2), and 25(OH)D levels were not associated with development of non-skin cancer (Table 2; Supplemental Figure). In the multivariable analysis, older age (per decade, HR 1.27; 95% CI, 1.15–1.41), CCI (HR 1.06; 95% CI, 1.01–1.12), and smoking history (HR 1.56; 95% CI, 1.25–1.95) were associated with development of non-skin cancer (Table 2). Compared with the reference group, only 25(OH)D <12 ng/mL (HR 1.56; 95% CI, 1.03–2.36), was significantly associated with increased risk of development of non-skin cancer (Table 2; Figure 1). In a sensitivity analysis excluding any cancer which developed within the first year of index 25(OH)D measurement, the hazard ratio for risk of developing non-skin cancer in the setting 25(OH)D <12 ng/mL remained consistent, but the analysis was underpowered to achieve statistical significance (HR 1.57, 95% CI 0.99–2.50, P=.06; data not shown). No increased risk of developing non-skin cancer was observed with 25(OH)D >50 ng/mL (Table 2; Figure 1).
We analyzed subjects who died from cancer separately (n=54). Cancer-related death was associated with age (HR 1.31; 95% CI, 1.07–1.60), non-white race (HR 3.91; 95% CI, 1.97–7.76), CCI (HR 1.20; 95% CI, 1.11–1.30), smoking history (HR 2.53; 95% CI, 1.35–4.74), and hypertension (HR 2.12; 95% CI, 1.18–3.79) on univariate analysis (Table 3). In the same analysis, 25(OH)D <12 ng/mL (HR 3.47; 95% CI, 1.60–7.49) and 25(OH)D 12–19 ng/mL (HR 2.76; 95% CI, 1.43–5.34) were associated with a significantly increased risk of cancer-related death compared with the reference group. In a multivariable model including race, CCI, smoking history, and baseline 25(OH)D status, CCI (HR 1.15; 95% CI, 1.06–1.25) and smoking history (HR 2.12; 95% CI, 1.12–4.02) were associated with cancer-related death (Table 3). In the same model, compared with the reference group, 25(OH)D <12 ng/mL (HR 2.35; 95% CI, 1.01–5.48) and 25(OH)D 12–19 ng/mL (HR 2.10; 95% CI, 1.05–4.22) were associated with a significantly increased risk of cancer-related death (Table 3; Figure 2).
Table 3.
Hazard ratios from Cox proportional hazards modeling of time from cancer diagnosis to time of death in those subjects who died from cancer.a
| Risk Factor | Univariate HR (95% CI) | Multivariable HR (95% CI) |
|---|---|---|
| Age (per decade) | 1.31 (1.07, 1.60) | |
| Male Sex | 1.33 (0.76, 2.33) | |
| Non-white Race | 3.91 (1.97, 7.76) | 2.19 (0.96, 4.96) |
| Charlson Comorbidity Index (per unit) | 1.20 (1.11, 1.30) | 1.15 (1.06, 1.25) |
| BMI (per kg/m2) | 1.03 (0.99, 1.06) | |
| HOUSES Z (per unit) | 0.92 (0.84, 1.00) | |
| Ever Smoked (Yes vs No) | 2.53 (1.35, 4.74) | 2.12 (1.12, 4.02) |
| Hypertension (Yes vs No) | 2.12 (1.18, 3.79) | |
| Osteoporosis/Osteopenia (Yes vs No) | 1.23 (0.72 (2.10) | |
| Vitamin D | ||
| 25(OH)D 20–50 ng/mL | 1.0 | 1.0 |
| 25(OH)D <12 ng/mL | 3.47 (1.60, 7.49) | 2.35 (1.01, 5.48) |
| 25(OH)D 12–19 ng/mL | 2.76 (1.43, 5.34) | 2.10 (1.05, 4.22) |
| 25(OH)D >50 ng/mL | 0.40 (0.05, 2.89) | 0.44 (0.06, 3.27) |
Time 0 is time of initial cancer diagnosis
Figure 2.
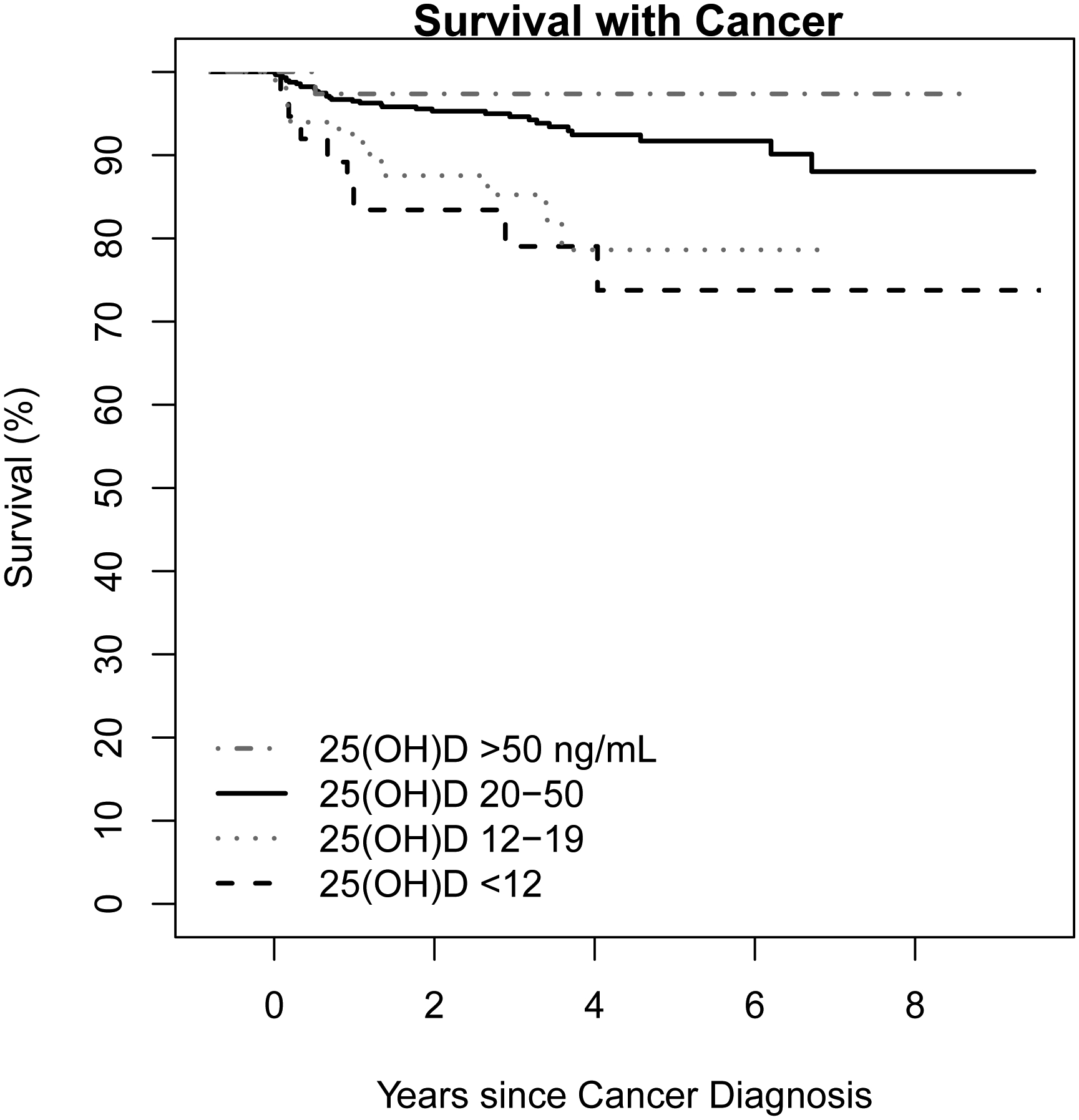
Kaplan-Meyer curve showing survival after cancer diagnosis in those subjects whose death was attributed to cancer. SI conversion factors: To convert 25(OH)D values to mmol/L, multiply by 2.496. 25(OH)D, 25-hydroxyvitamin D.
4. Discussion
In this population-based retrospective cohort study, low 25(OH)D status was associated with a greater subsequent incidence of non-skin cancers and cancer-specific mortality. Subjects with an index 25(OH)D measurement less than 12 ng/mL, after adjustment, were 1.6 times more likely to develop non-skin cancer than those with normal 25(OH)D levels. Moreover, among subjects who developed cancer, those with 25(OH)D levels of less than 12 ng/mL and 12–19 ng/mL were 3.5 and 2.8 times more likely to die from cancer, respectively, than the reference group.
Interestingly, there was no significant relationship between vitamin D and incidence of all combined cancer subtypes after adjustment. This finding emphasizes the importance of studies which evaluate the relationship between vitamin D and specific cancer subtypes, as vitamin D may play different roles in the biology of specific cancer etiology and progression. Our study results confirmed the inverse association between pre-diagnostic circulating 25(OH)D and invasive cancer incidence that has been reported across several observational studies.3–8, 19, 29 The question of causation in this apparent protective relationship of vitamin D and cancer remains unclear. One of the largest prospective cohort studies found that pre-diagnostic serum 25(OH)D correlated with incident colorectal cancer in women, but not incident breast or prostate cancers,19 a pattern that seems consistent across other reports.3, 30–33 A dose-response meta-analysis of vitamin D and colorectal cancer risk revealed that an increment of 16 ng/mL in circulating 25(OH)D corresponded with an increment odds ratio of 0.79 (95% CI 0.64–0.97).34 Conversely, increased risk of pancreatic cancer was observed with pre-diagnostic 25(OH)D levels greater than 40 ng/mL in a large case-control study.23 A meta-analysis of RCTs evaluating vitamin D supplementation and total cancer risk, showed a very small and insignificant trend (RR 0.98, 95% CI 0.93–1.03).16 Taken together, these data provide insight into discrepancies amongst reports of cancer incidence: Vitamin D appears to affect incident cancer subtypes differently.
Our study demonstrated a null association between vitamin D and skin cancer incidence after multivariate adjustment. However, the association trend appears to favor decreased skin cancer incidence among low vitamin D categories, and this relationship would indeed be expected given the known risk of skin cancer with sun exposure. Reports in the literature are conflicting. Pre-diagnostic 25(OH)D35 and vitamin D dietary intake36 had a null association with incident melanoma. Incident squamous cell carcinoma was associated with serum 25(OH)D37 but not vitamin D dietary intake,36 which is consistent with serum 25(OH)D being a proxy for ultraviolet light exposure.
We also confirmed an inverse association with vitamin D and cancer mortality.38 The cancer survival benefit afforded by vitamin D supplementation appears to be around 13–16%.16, 17, 39 These figures stand in stark comparison with our results, which suggest a twofold to threefold increase in cancer mortality with a low or very low vitamin D level. The reasons behind these mortality differences are likely multifactorial, reasons which overlap with the discrepancies regarding vitamin D and cancer incidence among observational studies and RCTs. Most notably, RCTs may have insufficient time to assess the relationship of vitamin D exposure and cancer incidence, and they often exclude those with vitamin D deficiency.16, 17 Vitamin D appears to be important for immune function,40 which has a critical role in regulation of carcinogenesis and progression. Hence, longstanding low vitamin D status may influence innate immunologic ability to prevent and fight cancer. Moreover, RCTs have often had unregulated vitamin D supplementation in control groups, which may moderate observed effects toward a null effect.15 Conversely, low vitamin D status may be a comorbid feature of poor overall health or an undiagnosed malignant or premalignant condition. While vitamin D supplementation appears to benefit cancer survival,16–18 cancer itself may also have an effect of decreasing vitamin D status. However, when we excluded subjects that developed cancer within 1 year of baseline 25(OH)D, we found that the association was unchanged.
Our study had several strengths. This was a large community-based population study with access to comprehensive medical, demographic, and individual-level socioeconomic data, included in the adjusted analysis. Measurement of serum 25(OH)D was performed in a single laboratory with isotope-dilution liquid chromatography-tandem mass spectrometry, providing optimal accuracy and standardization of measured values.
Several limitations of our study are also noteworthy. Due to the retrospective nature of the study, only patients who were clinically evaluated and underwent 25(OH)D measurement could be included, which may not accurately reflect the entire population. There was a predominance of women in the study, presumably having 25(OH)D measurement for evaluation of osteoporosis, which is more prevalent in women than in men. The adjustment for osteoporosis in the multivariable model should partially account for vitamin D supplementation in those with osteoporosis. We were unable to detect associations among individual cancer subtype and stage due to insufficient numbers of subtypes. Importantly, we could not establish causality in relation to vitamin D and cancer incidence and mortality due to the retrospective nature of the study. However, identifying a cohort free of cancer at the time of index vitamin D is the optimal retrospective design to overcome this limitation. In addition, it should be again emphasized that the association between low 25(OH)D levels and subsequent development of cancer, while real and significant, does not necessarily imply either causality or a direct role of vitamin D in the development of cancer. It is possible that vitamin D status is simply a biomarker of other (as-yet unidentified) covariates that are pathophysiologically related to the development of cancer. Finally, our results are based on one historic measurement of 25(OH)D, which may not be perfectly reflective of long-term vitamin D status or of subsequent vitamin D supplementation. However, serum 25(OH)D appears to be relatively constant over time.41
Future studies should aim to clarify discrepancies among reports of vitamin D and cancer incidence and mortality, including evaluation of individual cancer subtypes with larger cohorts. Randomized controlled trials evaluating vitamin D supplementation could benefit from prolonged vitamin D exposure and follow-up.
5. Conclusion
We demonstrated that pre-diagnostic serum 25(OH)D was inversely associated with incident non-skin cancer and cancer-related death. Serum 25(OH)D had a null association with incident skin cancer and total cancer incidence.
Supplementary Material
Acknowledgements:
This work was supported by CTSA Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding Information:
This study was funded by CTSA Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences. This study was also made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations and Acronyms:
- 25(OH)D
25-hydroxyvitamin D
- BMI
body mass index
- CI
confidence interval
- CCI
Charlson Comorbidity Index
- HR
hazard ratio
- HOUSES
HOUsing-based index of SocioEconomic Status
- RCTs
randomized controlled trials
- REP
Rochester Epidemiology Project
- SD
standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Casey Johnson, Daniel Dudenkov, Kristin Mara, Phil Fischer, Julie Maxson, and Tom Thacher declare they have no conflict of interest.
Ethical Approval: The study was approved by the Mayo Clinic Institutional Review Board, and all included records had research authorization for retrospective chart review.
6. References
- 1.Jin X, Xiong S, Ju S-Y, Zeng Y, Yan LL, Yao Y. Serum 25-Hydroxyvitamin D, Albumin, and Mortality among Chinese Older Adults: A Population-based Longitudinal Study. J Clin Endocrinol Metab. 2020;105(8):1–9. [DOI] [PubMed] [Google Scholar]
- 2.Fan X, Wang J, Song M, et al. Vitamin D status and risk of all-cause and cause-specific mortality in a large cohort: results from the UK Biobank [published online ahead of print July 4, 2020]. J Clin Endocrinol Metab. 2020. 10.1210/clinem/dgaa432. [DOI] [PubMed] [Google Scholar]
- 3.Garland C, Garland F, Shaw E, Comstock G, Helsing K, Gorham E. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet. 1989;2(8673):1176–1178. [DOI] [PubMed] [Google Scholar]
- 4.Gorham ED, Garland CF, Garland FC, et al. Vitamin D and prevention of colorectal cancer. J Steroid Biochem Mol Biol. 2005;97(1–2):179–194. [DOI] [PubMed] [Google Scholar]
- 5.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98(7):451–459. [DOI] [PubMed] [Google Scholar]
- 6.Ahonen MH, Tenkanen L, Teppo L, Hakama M, Tuohimaa P. Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland). Cancer Causes Control. 2000;11(9):847–852. [DOI] [PubMed] [Google Scholar]
- 7.Feskanich D, Ma J, Fuchs CS, et al. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2004;13(9):1502–1508. [PubMed] [Google Scholar]
- 8.Garland CF, Garland FC, Gorham ED, et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96(2):252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudenkov DV, Mara KC, Petterson TM, Maxson JA, Thacher TD. Serum 25-hydroxyvitamin D values and risk of all-cause and cause-specific mortality: A population-based cohort study. Mayo Clin Proc. 2018;93(6)721–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14(5):342–357. [DOI] [PubMed] [Google Scholar]
- 11.Laczmanski L, Laczmanska I, Lwow F. Association of select vitamin D receptor gene polymorphisms with the risk of tobacco-related cancers–a meta-analysis. Sci Rep. 2019;9(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai Y-H, Lu H, Hong D, Lin C-C, Yu Z, Chen B-C. Vitamin D receptor gene polymorphisms and colorectal cancer risk: a systematic meta-analysis. World J Gastroenterol. 2012;18(14):1672–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lurie G, Wilkens LR, Thompson PJ, et al. Vitamin D receptor gene polymorphisms and epithelial ovarian cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16(12):2566–2571. [DOI] [PubMed] [Google Scholar]
- 14.Guy M, Lowe LC, Bretherton-Watt D, et al. Vitamin D receptor gene polymorphisms and breast cancer risk. Clin Cancer Res. 2004;10(16):5472–5481. [DOI] [PubMed] [Google Scholar]
- 15.Falleti E, Bitetto D, Fabris C, et al. Vitamin D receptor gene polymorphisms and hepatocellular carcinoma in alcoholic cirrhosis. World J Gastroenterol. 2010;16(24):3016–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keum N, Lee D, Greenwood D, Manson J, Giovannucci E. Vitamin D supplementation and total cancer incidence and mortality: a meta-analysis of randomized controlled trials. Ann Oncol. 2019;30(5):733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Fang F, Tang J, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. 2019;366:l4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manson JE, Cook NR, Lee I-M, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380(1):33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heath AK, Hodge AM, Ebeling PR, et al. Circulating 25-hydroxyvitamin D concentration and risk of breast, prostate, and colorectal cancers: the Melbourne Collaborative Cohort Study. Cancer Epidemiol Biomarkers Prev. 2019;28(5):900–908. [DOI] [PubMed] [Google Scholar]
- 20.Zhu K, Knuiman M, Divitini M, et al. Lower serum 25-hydroxyvitamin D is associated with colorectal and breast cancer, but not overall cancer risk: a 20-year cohort study. Nutr Res. 2019;67:100–107. [DOI] [PubMed] [Google Scholar]
- 21.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ong J-S, Cuellar-Partida G, Lu Y, et al. Association of vitamin D levels and risk of ovarian cancer: a Mendelian randomization study. Int J Epidemiol. 2016;45(5):1619–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA, et al. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: cohort consortium vitamin D pooling project of rarer cancers. Am J Epidemiol. 2010;172(1):81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aul AJ, Dudenkov DV, Mara KC, Juhn YJ, Wi CI, Maxson JA, Thacher TD. The relationship of 25-hydroxyvitamin D values and risk of fracture: a population-based retrospective cohort study. Osteoporosis Int. 2020;31(9):1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melton LJ III. History of the Rochester epidemiology project. Mayo Clin Proc. 1996;71(3):266–274. [DOI] [PubMed] [Google Scholar]
- 26.Rocca WA, Yawn BP, Sauver JLS, Grossardt BR, Melton LJ. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.REP Population Overview. https://rochesterproject.org/for-researchers/population-overview/.AccessedJuly 1, 2020.
- 28.Juhn YJ, Beebe TJ, Finnie DM, et al. Development and initial testing of a new socioeconomic status measure based on housing data. J Urban Health. 2011;88(5):933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang D, Lei S, Wu Y, et al. Additively protective effects of vitamin D and calcium against colorectal adenoma incidence, malignant transformation and progression: A systematic review and meta-analysis. Clin Nutr. 2020;39(8):2525–2538. [DOI] [PubMed] [Google Scholar]
- 30.McCullough ML, Zoltick ES, Weinstein SJ, et al. Circulating vitamin D and colorectal cancer risk: an international pooling project of 17 cohorts. J Natl Cancer Inst. 2019;111(2):158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dimitrakopoulou VI, Tsilidis KK, Haycock PC, et al. Circulating vitamin D concentration and risk of seven cancers: Mendelian randomisation study. BMJ. 2017;359:j4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobs ET, Kohler LN, Kunihiro AG, Jurutka PW. Vitamin D and colorectal, breast, and prostate cancers: a review of the epidemiological evidence. J Cancer. 2016;7(3):232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mondul AM, Weinstein SJ, Layne TM, Albanes D. Vitamin D and cancer risk and mortality: state of the science, gaps, and challenges. Epidemiol Rev. 2017;39(1):28–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L, Zou H, Zhao Y, et al. Association between blood circulating vitamin D and colorectal cancer risk in Asian countries: a systematic review and dose-response meta-analysis. BMJ Open. 2019;9(12):e030513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai TY, Kuo CY, Huang YC. The association between serum vitamin D level and risk and prognosis of melanoma: A systematic review and meta analysis. J Eur Acad Dermatol Venereol. 2020;34(8):1722–1729. [DOI] [PubMed] [Google Scholar]
- 36.Park SM, Li T, Wu S, Li W-Q, Qureshi AA, Cho E. Vitamin D intake and risk of skin cancer in US women and men. PLoS One. 2016;11(8):e0160308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hosseini M-S, Salarvand F, Ehsani AH, et al. Relationship between level of serum 25-hydroxyvitamin D and risk of squamous cell carcinoma in an Iranian population. Dermatol Pract Concept. 2019;9(4):278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heath AK, Kim IY, Hodge AM, English DR, Muller DC. Vitamin D status and mortality: A systematic review of observational studies. Int J Environ Res Public Health. 2019;16(3):383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanellopoulou A, Riza E, Samoli E, Benetou V. Dietary Supplement Use after Cancer Diagnosis in Relation to Total Mortality, Cancer Mortality and Recurrence: A Systematic Review and Meta-Analysis [published online ahead of print March 9, 2020]. Nutr Cancer. 2020:1–15. 10.1080/01635581.2020.1734215. [DOI] [PubMed] [Google Scholar]
- 40.Vanherwegen A-S, Gysemans C, Mathieu C. Regulation of immune function by vitamin D and its use in diseases of immunity. Endocrinol Metab Clin North Am. 2017;46(4):1061–1094. [DOI] [PubMed] [Google Scholar]
- 41.McKibben RA, Zhao D, Lutsey PL, et al. Factors associated with change in 25-hydroxyvitamin D levels over longitudinal follow-up in the ARIC study. J Clin Endocrinol Metab. 2016;101(1):33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


