Abstract
Background
Since the declaration of COVID-19 pandemic, several case reports of demyelination of both peripheral and central nervous systems have been published. The association between CNS demyelination and viral infection has long been documented, and this link was recently reported following SARS-CoV-2 infection as well.
Objectives
In this systematic review, we aim to investigate the existing literature on CNS demyelination associated with SARS-CoV-2, and the proposed pathophysiological mechanisms.
Methods
We conducted a systematic review of articles in PubMed, SCOPUS, EMBASE, Cochrane, Google Scholar and Ovid databases, from 1 January 2020 until June 15, 2021. The following keywords were used: “COVID-19”, “SARS-CoV-2”, “demyelination”, “demyelinating disease”, “multiple sclerosis”, “neuromyelitis optica”, and “transverse myelitis”.
Results
A total of 60 articles were included in the final analysis of this systematic review and included 102 patients: 52 (51%) men and 50 (49%) women, with a median age of 46.5 years. The demyelination mimicked a variety of conditions with a picture of encephalitis/encephalomyelitis being the most common. At the same time other patterns were less frequently reported such as MS, NMOSD and even MOGAD. Longitudinally extensive transverse myelitis (LETM) was the most frequently reported pattern of spinal cord involvement.
Conclusion
A growing body of literature has shown an association between SARS‐CoV‐2 infection and the development of different types of CNS demyelination. Although causality cannot readily be inferred, this review may suggest a probable causal relationship, through a para-infectious or post-infectious immune-mediated etiology in COVID-19 patients. This relationship needs to be clarified in future research.
Keywords: COVID-19, SARS-CoV-2, Demyelinating disease, Multiple sclerosis
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan, China, in December 2019, and coronavirus disease 2019 (COVID-19) was declared a pandemic on March 11, 2020. Since then, several neurological manifestations have been reported, including both peripheral and central nervous systems (CNS) demyelinating diseases [1, 2]. Several articles reported the occurrence of acute demyelinating encephalomyelitis (ADEM), transverse myelitis (TM), multiple sclerosis (MS), and even neuromyelitis optica spectrum disorder (NMOSD), in association with COVID-19 infection [3–5].
There is an ongoing debate whether this association is merely related to the neurotropic features of SARS-CoV-2, or secondary to an acute or delayed immune-mediated response [6]. Coronavirus family showed clear evidence of neurotropic properties, as CNS demyelination has been reported with Middle East respiratory syndrome coronavirus (MERS-COV) and SARS-COV-1 [7–9].
When it comes to SARS-CoV-2, data are still limited in terms of cases of para-infectious and post-infectious CNS demyelinating/inflammatory diseases. Recent evidence has shown that SARS-CoV-2 can cross the blood–brain barrier and induce acute or delayed CNS demyelination [10]. Various mechanisms have been suggested including virus-induced hypercoagulable or proinflammatory states, direct viral invasion of the CNS, and post-infectious immune-mediated processes [11].
In this systematic review, we aim to investigate the available evidence regarding the different types of CNS demyelination in association with SARS-CoV-2 infection, and the proposed pathophysiological mechanisms in these cases.
Methods
Design
This systematic review collected data from PubMed, SCOPUS, EMBASE, Cochrane library, Google Scholar and Ovid databases, in accordance with Preferred Reporting Items for Systematic Reviews and Meta‐analysis (PRISMA) guidelines. We aimed to identify relevant articles that discussed CNS demyelination in association with SARS-CoV-2 infection from 1 January 2020 until June 15, 2021.
Search strategy
A pre-specified searching strategy consisted of a variation of keywords of relevant medical subject headings (MeSH) and keywords, including: “COVID-19”, “SARS-CoV-2”, “demyelination”, “demyelinating disease”, “multiple sclerosis”, “neuromyelitis optica”, and “transverse myelitis”. Furthermore, we hand-searched additional relevant COVID-19 articles that were referenced in the selected studies.
Inclusion criteria
We included all peer-reviewed publications that reported any form of CNS demyelination in association with COVID-19 infection, including but not limited to case reports and case series that met the following criteria: (i) studies reporting early or delayed acute CNS demyelination after COVID-19 infection; (ii) studies reporting possible association of cases fulfilling the diagnostic criteria of multiple sclerosis (MS), transverse myelitis (TM), neuromyelitis optica spectrum disorder (NMOSD), or myelin oligodendrocyte glycoprotein antibody disease (MOGAD), and COVID-19 infection; and (iii) studies published in English.
Exclusion criteria
The review was restricted to studies published in English. Publications that were not peer-reviewed were excluded from this study. We also excluded review papers, viewpoints, commentaries, unless reporting a case of demyelination, and studies where demyelination affected peripheral rather than central nervous systems. We also excluded cases not supported by positive imaging findings, laboratory or clinical evidence of COVID-19 infection.
Data extraction
Titles and abstracts of all identified studies were independently screened for relevance by the two reviewers, followed by full-text screening of the deemed eligible papers. The same reviewers then extracted data on the following parameters: article title, authors, publication year, age and gender of the patients, COVID-19-related information, onset of neurological symptoms, findings of neurological examination, MRI findings, laboratory work-up, CSF analysis, treatment and clinical outcome. Severity of COVID-19 infection was categorized into mild; asymptomatic infections or infections with mild symptoms not requiring hospitalization, moderate; requiring hospitalization but not ICU admission, and severe; requiring ICU admission and mechanical ventilation.
Statistical analysis
Qualitative data were described in percentages and numbers. Quantitative data were described using range (minimum and maximum), mean, standard deviation, and median. Significance of the obtained results was judged at the 5% level, but it could not be calculated due to insufficient data. A meta-analysis was planned to evaluate the association of the demographic findings, clinical, radiological and laboratory findings and outcomes, but it could not be performed due to lack of sufficient data.
Results
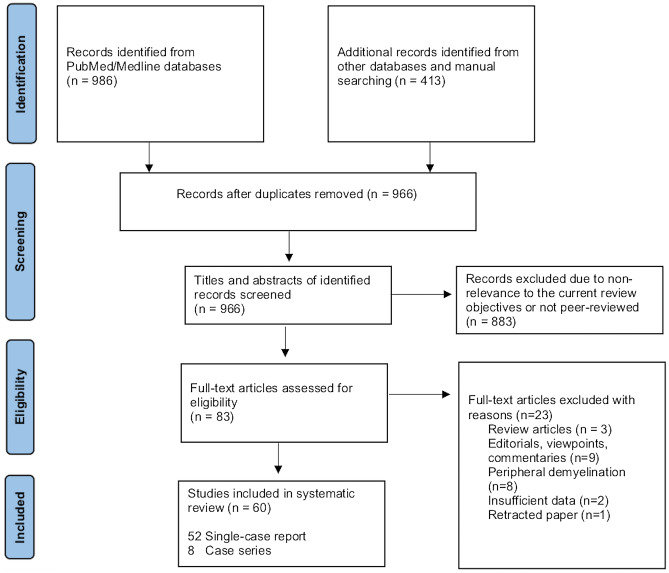
As illustrated in Fig. 1, our systematic search resulted in an initial number of 1,399 of potentially relevant articles, of which 966 were screened by title and abstract, following duplicates removal. Applying the inclusion/exclusion criteria to the full-text documents, 83 articles were deemed eligible, of which, 60 studies with a total of 102 patients, were included in the systematic review.
Fig. 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) study selection flow diagram
Brain demyelination
We identified 78 cases of brain demyelination from 38 articles. There were 40 males and 38 females. The median age of patients was 45.5 (0.17–77) years. The most common presenting neurological symptoms were lethargy, altered sensorium with or without seizures. In some cases, the symptoms followed COVID-19 infection by few weeks, while in others, they started at initial presentation and overlapped with COVID-19 symptoms. The demyelination mimicked a variety of conditions with the picture of encephalitis/encephalomyelitis being the most common (91%). At the same time, other patterns were less frequently reported such as MS, NMOSD and even MOGAD (10%).
It still remains an unanswered question whether demyelination represents a manifestation of direct viral invasion to the CNS, or it is an immune-mediated process triggering other well-known conditions, or in some cases a mere sequel for hypoxia affecting the CNS as a direct result of respiratory affection.
We summarized the results based on the most likely clinical diagnosis, in view of symptoms, laboratory and imaging findings in Tables 1 and 2.
Table 1.
Characteristics of cases presenting with COVID-19 related encephalitis/encephalomyelitis
| Author | Age (years) | Gender | Comorbidities | Time relation between SARS-COV-2 infection and NP | Presenting COVID-19 symptoms | Presenting neurological symptoms | Neurological diagnosis | Other NS manifestations | Treatment | Response to treatment | PCR testing for SARS-COV 2 | Severity of COVID-19 infection | Other laboratory investigations | MRI data |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Zoghi et al. [14] | 21 | Male | None | 2–3 weeks | Fever, chills, cough | Weakness and paresthesia of the lower limbs and upper limbs, urinary retention, vomiting, drowsiness and lethargy | ADEM vs NMOSD | None | PLEX for 5 days with antibiotics and antivirals | Partial improvement | Negative in nasopharynx and in CSF (Positive chest and IgG) | Mild | Negative OCBs |
Brain: bilateral corticospinal tracts, cerebral peduncle and pons, marbled hyperintensity in the splenium of corpus callosum Cervical spine: LETM |
| 2. Zanin et al. [32] | 54 | Female | NA | At initial presentation | Anosmia and ageusia | Loss of consciousness and seizures | HIE vs viral encephalitis, myelitis | None | High-dose steroid treatment (dexamethasone 20 mg/day for 10 days and 10 mg/day for 10 days) |
Marked improvement of pulmonary condition Neurological improvement: not mentioned |
Positive in nasopharynx, negative in CSF | Severe | NA |
Brain: hyperintensities PV, bulbo-medullary junction Cervicodorsal spine: patchy hyperintensities |
| 3. Brun et al. [33] | 54 | Female | HTN | 8 days | Fever, fatigue, respiratory distress | Hemiplegia and altered sensorium | HIE vs ADEM | None | Steroids | Partial improvement | Positive in nasopharynx, negative in CSF | Severe | NA | Brain: bilateral tumefactive demyelination, bilateral globus pallidi, DWM and corpus callosum, avid post-contrast enhancement |
| 4. Alqwaifly et al. [34] | 14 | Female | None | 2 weeks | Fever | Unsteadiness, left sided facial weakness | ADEM | None | IVMP 1 g for 5 days | Partial improvement | Positive in nasopharynx, negative in CSF | Mild | Negative OCBs | Brain: well-defined middle cerebellar peduncle lesion, faint post-contrast enhancement |
| 5. Paterson et al. [3] | 52 | Male | Asthma | 22 days | Fever, cough, dyspnea | Delayed recovery of consciousness after weaning from ventilation | ADEM | None | Supportive | Partial improvement | Positive in nasopharynx, negative in CSF | Severe | Negative OCBs | Brain: multiple lesions in DWM. Cyst-like areas of varied sizes, some with hemorrhagic foci and peripheral rims of restricted diffusion |
| 6. Paterson et al. [3] | 60 | Male | DM, HTN | 27 days | Fever, myalgia, fatigue, dyspnea | Altered consciousness | ADEM | None | IVMP 1 g for 3 days | Partial improvement | Negative PCR in CSF | Severe | Negative OCBS | Brain: multifocal and confluent areas of signal change in the cerebral DWM with extensive microhemorrhages in the subcortical regions |
| 7. Paterson et al. [3] | 66 | Female | HTN, hypothyroidism, hysterectomy, osteoarthritis, degenerative spine disease | 1 day | Fever | Confusion and seizures | Autoimmune/limbic encephalitis | None | IVMP 1 g for 3 days, then oral prednisolone taper, IVIG | Partial improvement | Negative in nasopharynx | Mild | Negative OCBs | Brain: T2-hyperintense signals in upper pons, limbic lobes, medial thalami and subcortical cerebral white matter |
| 8. Paterson et al. [3] | 59 | Female | Aplastic anemia, MGUS, breast cancer, fatty liver, hypercholesterolemia | 10 days | Couch, chills, lethargy, myalgia | GTCs and low conscious level | ADEM | None | Intubation, ventilation; levetiracetam, acyclovir and ceftriaxone, dexamethasone | No response, died | Negative in CSF | Mild | NA | Brain: extensive confluent, largely symmetrical areas in brainstem, limbic, and insular lobes, superficial subcortical white matter and deep grey matter, clusters of microhemorrhages, restricted diffusion and peripheral rim enhancement |
| 9. Paterson et al. [3] | 52 | Male | None | At presentation | Fever, respiratory distress | History of GBS 3 days before CNS symptoms. Increased weakness, dysphagia, ophthalmoplegia and altered sensorium | ADEM | AIDP | Intubation and ventilation, IVMP 1 g for 5 days, IVIG | Partial improvement | Positive in nasopharynx, negative in CSF | Severe | NA | Brain: multifocal confluent lesions in internal and external capsules, splenium and DWM of cerebral hemispheres. Over 5 days, lesions increased in size and showed multiple microhemorrhages and extensive prominent medullary veins. Spine: components of brachial and lumbosacral plexus showed increased signal and enhancement |
| 10. Paterson et al. [3] | 47 | Female | Asthma | 8 days | Cough, fever, shortness of breath | Headache, left-hand numbness, left sided facial weakness, left upper limb weakness and mild left leg weakness, reduced conscious level | ADEM | None | Intubation, hemicraniectomy, IVMP 1 g for 5 days, oral prednisolone, IVIG | Partial improvement | Negative PCR in brain tissue | Severe | NA | Brain: severe right hemispheric vasogenic oedema with a leading edge on contrast imaging. Smaller areas of T2-hyperintense changes in the left hemisphere. Marked mass-effect |
| 11. Paterson et al. [3] | 54 | Female | HTN, PCOS | 14 days | Cough, fever, dysgeusia, rash | Unsteadiness, left sided weakness, slurred speech, fatigue and falls | ADEM | None | IVMP 1 g for 3 days, then oral prednisolone | Partial improvement | NA | Mild | Negative OCBs | Brain: multiple large lesions with peripheral rim restriction in periventricular white matter of both cerebral hemispheres |
| 12. Paterson et al. [3] | 60 | Female | DM, HTN | 18 days | Fever, cough, dyspnea, diarrhea | Delayed recovery in ICU | ADEM | None | IVMP 1 g for 3 days, then oral prednisolone taper. Intubation and ventilation; renal replacement | Partial improvement | Negative in CSF | Severe | Negative OCBs | Brain: multifocal lesions with diffusion changes in PV white matter and corpus callosum |
| 13. Paterson et al. [3] | 33 | Female | None | 2 days | Fever | Headache, confusion, reduced conscious level | ADEM, LETM | None | Intubation, ICP bolt; lumbar drain; IVMP 1 g for 3 days then oral prednisolone | Partial improvement | Negative in CSF | Mild | Negative OCBs, MOG, AQP4 antibodies |
Brain: multifocal lesions in lower brainstem, medial temporal lobes and DWM, some of which showed restricted diffusion. 3 days later, the brainstem lesions coalesced and extensive intramedullary lesions Spine: oedema involving grey and white matter of the spinal cord appeared |
| 14. Paterson et al. [3] | 27 | Female | None | 8 days | Fever, cough, anosmia, dysgeusia | Sensory symptoms in feet and right hand; difficulty with balance and walking | ADEM, TM | None | None | Complete recovery | NA | Mild | NA |
Brain: diffuse ill-defined confluent T2-hyperintensity involving the white matter of the cerebral hemispheres, largely along the corticospinal tracts. Small focal area of diffusion changes in the left motor cortex Spine: ill-defined intramedullary lesion without swelling in the conus medullaris |
| 15. Poyadji et al. [35] | 58 | Female | None | At initial presentation | Fever, cough | Altered sensorium | ADEM | None | IVIG | NA | Positive in nasopharynx | NA | NA | Brain: hemorrhagic rim-enhancing lesions within the bilateral thalami, medial temporal lobes, and subinsular regions |
| 16. Varadan [36] | 46 | Male | Alcoholic liver disease | 5 weeks | Fever, dyspnea | Headache, left hemiplegia, left facial and altered mental status | AHLE | None | IVMP 1 g for 5 days | Deteriorated and died | Positive in nasopharynx | Moderate | NA | Brain: T2-hyperintense white matter lesions in bilateral frontal, parietal lobes, left thalamus, left cerebral peduncle, and medulla. Internal areas of diffusion restriction and irregular patchy areas of rim enhancement were noted within most of the lesions. Left parietal PV white matter lesion was reaching the ventricular atrium with subjacent faint subependymal enhancement. Few microbleeds were seen |
| 17. Yong MH et al. [37] | 61 | Male | DM, HTN, hyperlipidemia | 20 days | Fever, cough, anosmia, respiratory failure | Encephalopathy | AHL vs ANE | None | Remdesivir, enoxaparin, mannitol, PLEX, IVIG | Tetra paretic and dysphasic at time of writing | Positive in nasopharynx | Severe | NA | Brain: multifocal subcortical white matter lesions in bilateral cerebral hemispheres with associated petechial hemorrhages and vasogenic edema. Bilateral thalamic and cerebellar involvement present. Incomplete ring-like enhancement surrounded the thalamic lesions |
| 18. Alan Chalil et al. [38] | 48 | Female | None | 2 weeks | Myalgia, dry cough, dyspnea, fever | Altered consciousness | AHLE | None | Vasopressor and steroids | Partial improvement | Positive in nasopharynx, negative in CSF | Severe | NA | Brain: extensive bilateral parietal and occipital intraparenchymal hemorrhage, with surrounding edema with intraventricular extension and acute hydrocephalus, cortical enhancement |
| 19. Karapanayiotides T et al. [39] | 57 | Male | None | 3 days | Fever, cough | Altered sensorium | AHLE | None | Azithromycin, hydroxychloroquine and lopinavir/- ritonavir, anakinra | Partial improvement | Positive in nasopharynx, negative in CSF | Severe | NA | Brain: bilateral subacute hemorrhagic lesions in the basal ganglia with perilesional edema and hemorrhage. Insular, temporal and frontal lobe white matter involvement with concentric demyelination pattern |
| 20. Handa R et al. [40] | 33 | Male | CKD, HTN | At initial presentation | Fever | Progressive weakness of upper and lower limbs and altered sensorium, seizures | AHLE, myelitis | None | IVMP 1 g | Partial improvement then death due to respiratory failure | Positive in nasopharynx | Severe | NA | Brain and spine: bilateral frontoparietal and subcortical hyperintensities affecting splenial, medullary and cervical cord involvement with petechial hemorrhage and splenial diffusion restriction |
| 21. Ghosh et al. [41] | 44 | Female | None | 2 days after fever resolution | High-grade fever, myalgia dry cough, hypogeusia, hyposmia | Confusion, disorientation, GTCs, loss of sphincter control and loss of consciousness | AHNE | None | IVMP 1 g for 5 days | Died | Positive in nasopharynx | Mild | Elevated IgG index | Brain: limited MR images showing left frontoparietal and right parietal white matter lesions with hemorrhage and edema |
| 22. Haqiqi et al. [42] | 56 | Male | HTN, CKD, hypercholesterolemia, asthma | 7 days | Flu-like symptoms | Altered sensorium | AHLE | None | Supportive | No improvement | Positive in nasopharynx, negative in CSF | Severe | Positive OCBs | Brain: symmetrical signal with hemosiderin staining. Cystic hemorrhagic areas with fluid levels. Areas of restricted diffusion. Slight partial resolution of findings in repeat MRI |
| 23. Mullaguri et al. [43] | 77 | Female | Parkinson's disease, cognitive impairment, and HTN | At initial presentation | Fever, fatigue, disorientation, shortness of breath | Disturbed sensorium | AHNE | None | Supportive | No improvement, died | Positive in nasopharynx | Severe | NA | Brain: tiny foci of restricted diffusion involving bilateral centrum semiovale and inferior left cerebellar hemisphere. SWI revealed innumerable areas of microhemorrhages in the bilateral cerebral hemispheres involving the corona radiata, centrum semiovale, internal capsule, globus pallidus, the gray–white junction of all lobes, pons, bilateral middle cerebellar peduncles, and cerebellar hemispheres |
| 24. Mullaguri et al. [43] | 68 | Female | CLL, HTN | At initial presentation | Malaise, nausea, diarrhea, progressive dyspnea, high-grade fever | Encephalopathy | AHNE | None | Supportive | No improvement, died | Positive in nasopharynx | Severe | NA | Brain: T2/FLAIR hyperintense PV lesions with diffusion restriction involving the bilateral centrum semiovale, right internal capsule, left parietal cortex, and bilateral cerebellum. SWI demonstrated multiple areas of microhemorrhages in the bilateral cerebral cortex, basal ganglia, and cerebellar hemispheres |
| 25. Radmanesh et al. [44] | 11 patients mean age, 53 years; age range, 38–64 years | 9 males, 2 females | NA | NA | NA | Altered mental status | ANE | NA | NA | No improvement, died | NA | Severe | NA | Brain: diffuse leukoencephalopathy; symmetrical, confluent T2-hyperintensities with mild restricted diffusion, and involved bilateral deep and subcortical white matter. Infratentorial parenchyma tended to be less affected; only four patients had mild involvement of middle cerebellar peduncles and medial cerebellar hemispheres |
| 26. Sachs et al. [45] | 59 | Male | Asthma | NA | Fever, upper respiratory symptoms | NA | Hemorrhagic leukoencephalopathy | None | NA | NA | Positive in nasopharynx | Severe | NA | Brain: diffuse, confluent T2/FLAIR hyperintensities in posterior predominant white matter with scattered microhemorrhages predominantly in the corpus callosum, and apparent posterior circulation without diffusion restriction or abnormal enhancement |
| 27. McLendon et al. [46] | 1.4 | Female | None | 13 days | Fever | Progressive fatigue, decreased communication, difficulty feeding and walking, without support. Parental complaint of irritability, weakness of upper extremities, and gait disturbance, seizures | ADEM | None | IVIG 2 g/kg for four days, IVMP (30 mg/kg/day) for 5 days | Complete recovery after 2 months | Positive in nasopharynx and positive IgG antibodies | Mild | Negative OCBs, normal IgG index | Brain: multifocal hyperintense T2/FLAIR signals in bilateral subcortical and PV white matter without contrast enhancement |
| 28. Scullen et al. [47] | 63 | Female | HTN, obesity | 6 days | Fever, cough, shortness of breath, chest pain | Encephalopathy | HIE | None | Glucocorticoids | NA | Positive in nasopharynx | Severe | NA | Brain: FLAIR changes and diffusion restriction in bilateral globus pallidi and bilateral centrum semiovale. Gradient echo showed subtle changes in bilateral globus pallidi |
| 29. Scullen et al. [47] | 43 | Female | HTN, DM | 2 weeks | Cough, dyspnea | Encephalopathy | ANE | None | PLEX | No improvement | Positive in nasopharynx | Severe | NA | Brain: FLAIR changes in bilateral mesial temporal structures, lenticular nuclei, crus cerebri, and centrum semiovale with diffusion- restriction of those areas together with the splenium, body, and genu of the corpus callosum. SWI sequences showed hemorrhagic conversion in the left cerebral peduncle and bilateral basal ganglia |
| 30. Virhammar et al. [48] | 55 | Female | None | 7 days | Fever, myalgia | Lethargy and disturbed sensorium, multifocal myoclonus | ANE | None | IVIG and acyclovir, PLEX | Partial improvement | Positive in nasopharynx, initially negative in CSF, later positive in 3rd sample | Mild | NA | Brain: symmetrical pathological signal pattern in all sequences. Partial regression in follow-up |
| 31. Dixon et al. [49] | 59 | Female | Aplastic anemia | 10 days | Fever, cough and headache, myalgia, dyspnea | Seizure and altered sensorium | ANE | None | Acyclovir, supportive treatment, high-dose dexamethasone | No improvement, died | Positive in nasopharynx, negative in CSF | Mild | NA | Brain: extensive, relatively symmetrical changes throughout the supratentorial and infratentorial areas with diffuse swelling and hemorrhage in the brain stem and amygdalae. Extensive abnormal signal and microhemorrhage within thalamic nuclei, subinsular regions, splenium of corpus callosum, cingulate gyri, and subcortical perirolandic regions with restricted diffusion with peripheral enhancement, tonsillar herniation |
| 32. Montes-Ramirez [50] | 27 | Female | None | 17 days | Fever, dyspnea | Aphasia and quadriparesis | Diffuse leukoencephalopathy a with microbleeds | None | IVMP | Complete recovery | Positive in nasopharynx | Severe | NA | Brain: ependymal enhancement, leukoencephalopathy, and microbleeds |
| 33. McCuddy et al. [51] | 37 | Female | DM, HTN, obesity | 22 days | Fever, cough, chest pain, dyspnea | Diffuse weakness post- extubation | ADEM | None | Decadron 20 mg IV for 5 days, 10 mg IV for 5 days | Marked partial improvement | Negative CSF, positive in serum | Severe | Negative OCBs | Brain: T2-hyperintensity with restricted diffusion in corpus callosum, bilateral cerebral WM, pons, bilateral ventral medulla, with some enhancement |
| 34. McCuddy et al. [51] | 56 | Male | DM, HTN, CKD, asthma | 20 days | Fever, cough, chest pain and dyspnea | Encephalopathy | ADEM | None | Solumedrol 1 g for 5 days, IVIG | Mild partial improvement | Negative in CSF, positive in serum | Severe | Negative OCBs | Brain: diffuse hyperintensities in cerebral WM and cerebellum, with restricted diffusion |
| 35. McCuddy et al. [51] | 70 | Female | DM, HTN, CKD, obesity | 16 days | Fever, cough, chest pain, dyspnea | Encephalopathy | ADEM | None | Solumedrol 1 g for 5 days, IVIG | Mild partial improvement | Negative in CSF, positive in serum | Severe | Negative OCBs | Brain: T2-hyperintensities in cerebral WM, corpus callosum, brachium pontis with some restricted diffusion |
| 36. Assuncao et al. [52] | 49 | Male | None | 30 days | Respiratory symptoms | Altered sensorium | ADEM | None | NA | NA | Positive in nasopharynx, negative in CSF | Severe | NA | Brain: an unusual DWI pattern with nodular and ring-shaped lesions involving the PV and DWM |
| 37. Assuncao et al. [52] | 9 | Male | None | 37 days | None | Difficulty walking and speaking, right hemiparesis, and impaired ocular motor function | ADEM | None | NA | NA | Positive serology | Mild | NA | Brain: unusual DWI pattern with nodular and ring-shaped lesions involving the PV and DWM |
| 38. Parsons et al. [53] | 51 | Female | NA | At initial presentation | Fever, dyspnea, vomiting | Altered sensorium | ADEM | None | IVMP 1 g for 5 days, IVIG for 5 days | Partial improvement | Positive in nasopharynx, negative in CSF | Severe | Negative OCBs, AQP4 antibodies | Brain: scattered hyperintense lesions on FLAIR imaging in DWM and juxta-cortical areas, left frontal juxta-cortical white matter showed mild enhancement with a small amount of intraventricular hemorrhage in the occipital horns of both lateral ventricles. No parenchymal hemorrhage |
| 39. Langley et al. [54] | 53 | Male | NA | 8 days | Fever, cough, shortness of breath, myalgia, malaise | Altered sensorium | ADEM | None | IVMP 1 g for 3 days followed by two 500 mg doses | Partial improvement | Positive nasopharynx | Severe | Positive OCBs | Brain: multiple hyperintense lesions within the subcortical and DWM of the frontoparietal lobes bilaterally with restricted diffusion centrally. No leptomeningeal enhancement was seen. Small intraventricular hemorrhage within the occipital horns of the lateral ventricles. SWI showed microhemorrhages in parietal gyri, bilateral superior frontal lobes and occipital lobe |
| 40. Wong et al. [55] | 40 | Male | None | 13 days | Fever, dyspnea on exertion | Unsteady gait, diplopia, oscillopsia, limb ataxia, altered sensation in right arm, hiccups and dysphagia | Inflammatory brain stem encephalitis, LETM | None | Supportive | Partial improvement | Positive in nasopharynx | Moderate | NA |
Brain: hyperintensities in right inferior cerebellar peduncle, extending to involve a small portion of cervical cord with associated microhemorrhage The supratentorial region of the brain was normal Spine: LETM measuring 28 mm in longitudinal extent |
| 41. Novi et al. [56] | 64 | Female | Vitiligo, HTN, MGUS | 2 weeks | Influenza-like symptoms, anosmia, ageusia | Irritability and bilateral vision impairment associated with sensory deficit on her right leg | ADEM, TM | None | IVMP 1 g for 5 days tapered with oral prednisone 75 mg/d) associated with IVIG (2 g/kg in 5 days) | Marked but partial improvement | Negative in nasopharynx, positive in CSF | Mild | Negative OCBs |
Brain: evidence of multiple T1 post-Gd enhancing lesions of the brain Spine: single spinal cord lesion at the T8 level Orbit: bilateral optic nerve enhancement |
| 42. Otluoglu et al. [57] | 48 | Male | None | At initial presentation | Progressive headache and persistent cough, fatigue, myalgia, anosmia | Headache, anosmia | Viral encephalitis, TM | None | IVMP 1 g for 5 days, IV acyclovir for 21 days | NA | Negative in nasopharynx, positive in CSF | Mild | NA |
Brain: hyperintense lesions both in the posterior medial cortical surface of the temporal lobe consistent with viral encephalitis Spine: hyperintense lesions confined to the upper cervical spinal cord |
| 43. Utkuri et al. [58] | 44 | Male | None | At initial presentation | None | Urinary retention for 2 days, bilateral lower limbs weakness and numbness, inability to walk, lethargy, dysarthria and upper limb ataxia | ADEM, LETM | None | IVMP 1 g, IVIG | Partial improvement | Positive in nasopharynx, negative in CSF | Mild | Negative OCBs, normal IgG index |
Brain: PV and juxta-cortical lesions with homogeneous brisk enhancement in the left parietal lobe juxta-cortical/cortical lesions Spine: non-enhancing T2 hyperintense lesions throughout cervical and thoracic. Slight expansion of the conus medullaris with mild T2 hyperintensity and minimal foci of enhancement |
| 44. Lopes et al. [59] | 59 | Female | HTN | 3 days | Fever, cough, dyspnea, respiratory failure | Disturbed level of consciousness | ADEM | None | Non-specific | Died | Positive in nasopharynx, negative in CSF | Severe | Negative OCBs | Brain: multiple bilateral focal areas of signal abnormalities in the cerebral and cerebellar white matter, including corpus callosum, cerebellar and globus pallidus |
| 45. Lopes et al. [59] | 41 | Male | DM, HTN, obesity | 6 days | Fever, rhinorrhea, progressive dyspnea, respiratory failure | Disturbed level of consciousness | ADEM | Sensorimotor polyneuropathy | NA | Marked improvement | Positive in nasopharynx, negative in CSF | Severe | Negative OCBs | Brain: focal lesions located in the centrum semiovale, bilaterally, right thalamus, globus pallidus bilaterally, and anterior limb of internal capsule |
| 46. Lindan et al. [29] | 0.17 | Male | None | 1 day | Fever, cough | Seizures | ADEM | None | Supportive measures in ICU, AEDs | Marked improvement | Positive in nasopharynx | Severe | NA | Brain: multifocal T2 hyperintensity throughout bilateral thalami |
| 47. Lindan et al. [29] | 1.17 | Female | None | 2 days | Fever | Encephalopathy, dystonic posturing, seizures | ADEM | None | Intubated, supportive measures in ICU, AEDs | Marked improvement | Positive in nasopharynx | Severe | NA | Brain: confluent areas of T2 hyperintensity and restricted diffusion in the central gray, pons, and subcortical white matter. Splenial lesion |
| 48. Lindan et al. [29] | 9 | Male | Asthma | 5 days | Fever, cough, headache | Encephalopathy, photophobia, phonophobia, seizures | ADEM | None | Intubated, supportive measures in ICU, IVIG | Marked improvement | Positive in nasopharynx | Severe | NA | Brain: diffuse leptomeningeal enhancement, patchy T2 hyperintensity of cerebral white matter and cerebellum. Cortical, thalamic and splenial signal abnormalities. Follow-up 2.5 weeks: extension to optic chiasm and bilateral pyramidal tracts |
| 49. Lindan et al. [29] | 13 | Male | None | 1 day | Fever, headache | Lower limb weakness | ADEM, LETM | None | IVMP | Marked improvement | Positive in nasopharynx | Mild | NA |
Brain: extensive patchy white matter and basal ganglia T2 hyperintensities, associated mass effect and mild enhancement of right frontal lobe Spine: long segment mildly expansive central cord T2 hyperintensity. No post-contrast imaging of spine |
| 50. Lindan et al. [29] | 0.25 | Male | None | 12 days | Cough without fever, | Lower > upper limb spasticity and brisk DTR’s, reduced weight bearing | ADEM, LETM | None | IVIG | Partial improvement | Positive in nasopharynx | Mild | NA |
Brain: T2 hyperintensity brainstem Spine: long segment cord T2 hyperintensity with central gray matter predominance. No post-contrast imaging |
| 51. Lindan et al. [29] | 1.58 | Female | None | 3 days | Irritability | Gait impairment, constipation | ADEM, LETM | Neuritis: cauda equina | High-dose steroids | Complete improvement | Positive in nasopharynx | Mild | NA |
Brain: punctate and linear T2 hyperintense foci and enhancement in subcortical white matter Spine: long segment T2 hyperintensity with central gray matter predominance and patchy enhancement. Enhancement of cauda equina |
| 52. Lindan et al. [29] | 14 | Female | None | 2 days | Fever | Encephalopathy, seizures, respiratory failure | ADEM | Anti-NMDAR autoimmune encephalitis | Intubated, supportive measures in ICU | No improvement | Positive in nasopharynx | Severe | NA | Brain: initial normal, follow-up 4 weeks: patchy T2 hyperintensity white matter and basal ganglia, brainstem, cerebellar peduncles. No restricted diffusion or enhancement |
| 53. Lindan et al. [29] | 5 | Female | None | 1 day | MIS-C syndrome, fever, abdominal pain, diarrhea | Encephalopathy, | ADEM | None | Supportive measures in ICU | Complete improvement | Positive in nasopharynx | Severe | NA | Brain: T2 hyperintensity cerebral white WM. Focal CC and splenial lesions of corpus callosum, T2 hyperintensity and restricted diffusion |
| 54. Lindan et al. [29] | 9 | Male | None | 11 days | MIS-C syndrome | Encephalopathy, gait impairment | ADEM | Myositis | Supportive measures in ICU | Complete improvement | Positive in nasopharynx | Severe | NA | Brain: T2 hyperintensity cerebral white WM |
| 55. Lindan et al. [29] | 9 | Male | None | 2 days | MIS-C syndrome, fever, headache, neck pain | Encephalopathy, cerebellar signs, weakness | ADEM | Myositis | Supportive measures in ICU | Partial improvement | Positive in nasopharynx | Severe | NA | Brain: T2 hyperintensity cerebral WM. Splenial lesion T2 hyperintensity and restricted diffusion |
| 56. Lindan et al. [29] | 13.25 | Female | None | 1 day | MIS-C syndrome, fever | Headache encephalopathy, facial paralysis | ADEM | Neuritis | Supportive measures in ICU | Partial improvement | Positive in nasopharynx | Severe | NA | Brain: T2 hyperintensity hypothalamus. Bilateral neuritis CN VII |
| 57. Lindan et al. [29] | 13.83 | Female | Asthma | 6 days | MIS-C syndrome with cardiac dysfunction, | Stupor, pyramidal signs | ADEM, TM | None | Supportive measures in ICU | Complete improvement | Positive in nasopharynx | Severe | NA |
Brain: T2 hyperintensity hypothalamus Spine: focal T2 hyperintensity in thoracic cord with central predominance |
| 58. Lindan et al. [29] | 14.5 | Male | None | 8 days | MIS-C syndrome, fever, diarrhea, rash, hypotension | Encephalopathy, | ADEM | None | Supportive measures in ICU | Complete improvement | Positive in nasopharynx | Severe | NA | Brain: T2 hyperintensity cerebral WM. Splenial lesion T2 hyperintensity and restricted diffusion |
| 59. Lindan et al. [29] | 15 | Female | Obese | 18 days | MIS-C syndrome, fever, cough, dyspnea | Encephalopathy, myalgias, leg swelling | ADEM | Vasculitis/thrombosis | Supportive measures in ICU | Complete improvement | Positive in nasopharynx | Severe | NA | Brain: T2 hyperintensity cerebral WM. Splenial lesion T2 hyperintensity and restricted diffusion Innumerable microthrombi cerebrum, brainstem, cerebellum |
| 60. Lindan et al. [29] | 0.83 | Male | None | 1 week | Fever | Right ptosis, hypotonia, encephalopathy | ADEM, LETM | Neuritis | Supportive measures, high-dose steroids | Partial improvement. Relapse at 3 months after weaning steroids | Negative PCR in nasopharynx, serology positive | Severe | NA |
Brain: patchy T2 hyperintensities in cerebral WM, thalami, brainstem and cerebellum. Associated foci of enhancement and restricted diffusion Spine: long segment cord T2 hyperintensity with central gray predominance and without enhancement |
| 61. Lindan et al. [29] | 4.17 | Male | None | NA | Skin rash | Seizures, facial palsy, four limb dysfunctions | ADEM | None | High-dose steroids | Marked improvement | Negative PCR in nasopharynx, | Mild | Anti-MOG positive | Brain: T2-hyperintense lesions in cerebral cortex and thalamus. No enhancement |
ADEM acute disseminated encephalomyelitis, TM transverse myelitis, NMOSD neuromyelitis optica spectrum disorders, HIE hypoxic ischemic encephalopathy, LETM longitudinally extensive myelitis, AHLE acute hemorrhagic necrotizing encephalitis, ANE acute necrotizing encephalitis, AHNE acute hemorrhagic necrotizing encephalitis, OCBs oligoclonal bands, CSF cerebrospinal fluid, DM diabetes mellitus, HTN hypertension, IVMP intravenous methylprednisolone, GTCs generalized tonic–clonic convulsions, CNS central nervous system, GBS Guillain–Barre syndrome, AIDP acute inflammatory demyelinating polyneuropathy, MOG myelin oligodendrocyte glycoprotein, AQP4 Aquaporin4, PCR polymerase chain reaction, CLL chronic lymphocytic leukemia, IgG immunoglobulin G, PLEX plasma exchange, CKD chronic kidney disease, WM white matter, CC corpus callosum, MGUS monoclonal gammopathy with unknown significance, PCOS polycystic ovarian syndrome, PV periventricular, DWM deep white matter, SWI susceptibility-weighted imaging, FLAIR fluid-attenuated inversion recovery, ICU intensive-care unit, AEDs anti-epileptic drugs, NMDAR N-methyl D-aspartate receptor, MIS-C multisystem inflammatory syndrome in children
Table 2.
Characteristics of cases presenting with other COVID-19 related demyelinating syndromes
| Author | Age | Gender | Comorbidities | Time relation between SARS-COV2 infection and NP | Presenting COVID symptoms | Presenting neurological symptoms | Neurological diagnosis | Other NS manifestations | Treatment | Response to treatment | Testing for SARS-COV 2 | Severity of COVID infection | Antibody and OCBs testing | MRI data |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Moore et al. [12] | 28 | Male | Glaucoma and right retinal hole treated with laser ablation | 2 weeks | Fever, myalgia, anosmia, sore throat, headache and cough | Diplopia, vertigo, right oral numbness | MS | None | 3 days pulse steroids with oral taper | Partial improvement | Positive nasopharyngeal PCR | Mild | Positive OCBs | Brain MRI: juxta-cortical, PV and infratentorial lesions |
| 2. Palao et al. [60] | 29 | Female |
Asthma Rhino conjunctivitis |
2–3 weeks | Anosmia and dysgeusia asthenia and proximal myalgia in her limbs | Right optic neuritis | MS | None | 3 days pulse steroids with oral taper | Partial improvement |
Negative PCR in nasopharynx and CSF Positive IgM and IgG |
Mild | Positive OCBs |
Orbital MRI: right optic nerve lesion with contrast enhancement Brain MRI: sparse supratentorial PV demyelinating lesions |
| 3. Yavari et al. [5] | 24 | Female | None |
1 month after onset Overlapped symptoms |
Sore throat, low-grade fever, myalgia, anosmia | Blurred vision, diplopia, left lower motor neuron facial palsy, paresthesia of fingertips of both arms | MS-like | None |
4 days pulse steroids INF-beta 1a: 3 times weekly |
Partial improvement | Positive PCR in nasopharynx | Mild | NA | Brain MRI: atypical patches in the subcortical and DWM |
| 4. de Ruijter [61] | 15 | Male | None | Few weeks | Fever, nausea and cough | Bilateral optic neuritis | MOGAD (bilateral ON) | None | Pulse steroids | Almost complete recovery in 2 weeks | NA | Mild |
Negative OCBs Negative AQP4 Positive MOG antibody |
Orbital MRI: bilateral extensive optic neuritis |
| 5. Zoghi et al. [14] | 21 | Male | None | 2–3 weeks from onset of COVID symptoms | Fever, chills, non-productive cough | Weakness and paresthesia of the lower limbs and upper limbs, urinary retention, vomiting and drowsiness and lethargy | ADEM vs NMOSD | None | PLEX for 5 days with antibiotics and antivirals | Partial improvement | Negative PCR in nasopharynx and in CSF (positive chest and IgG) | Mild |
Negative OCBs Negative AQP4 antibody Negative MOG antibody |
Brain MRI: bilateral corticospinal tracts up to cerebral peduncle and pons, marbled hyperintensity in the splenium of corpus callosum Cervical spine MRI: LETM |
| 6. Correa et al. [4] | 51 | Female | None | 2 weeks | Fever, cough myalgia, headache, anosmia, ageusia | Dysesthesia, abdominal band (T6–10) associated with lower extremity numbness and dysesthesias, proprioceptive deficits, urinary retention, and left lower extremity weakness | NMOSD | Radiculitis |
5-day course of methylprednisolone (1 g/day) followed by plasma exchange For long- term attack prevention, she is using azathioprine 3 mg/kg per day |
Remarkable neurological improvement |
Positive PCR in nasopharynx Negative PCR in CSF |
Mild |
Serum and CSF cell-based assay for anti-AQP4 antibodies were positive Positive IgG index |
Brain MRI: a hyperintense lesion on T2WI and FLAIR in the anterior fornix and in the subfornical organ, with contrast enhancement Spinal MRI: (LETM) with a ring enhancement pattern and radiculitis |
| 7. Zhou et al. [62] | 26 | Male | None | Few days | Dry cough | Eye pain, visual loss, lower limb numbness | MOGAD (bilateral ON + myelitis) | None | Intravenous methylprednisolone for 5 days, followed by an oral prednisone taper |
Visual acuity Improved |
Positive nasal and oropharyngeal PCR | Mild |
Positive OCBs Positive (MOG) IgG Negative AQP4 antibody |
Spinal MRI: patchy T2 hyperintensities in the lower cervical and upper thoracic spinal cord associated with mild central thickening and GAD enhancement Brain and orbits MRI: avid, uniform enhancement and thickening of both optic nerves extending from the globe to their intracranial, pre-chiasmal segments, without overt involvement of the chiasm One small non-enhancing, nonspecific periventricular T2 hyperintensity was present, adjacent to the occipital horn of the right lateral ventricle |
| 8. Kogure et al. [63] | 47 | Male | Right adrenal resection. Recurrent paranasal sinuses | 2 days | Asymptomatic (close contact of a positive case) | Left eye pain and upper visual field defect | MOGAD | None | Methylprednisolone 1 g/day for a total of 3 days, followed by an oral prednisolone taper | Partial |
Negative PCR in nasopharynx Negative PCR in CSF |
Mild | Positive MOG antibody | Orbit MRI: post-contrast T1-weighted fat-suppressed MRI revealed the bilateral (but left-dominant) uniform enhancement along with optic nerve sheaths |
NP neurological presentation, NS nervous system, MS multiple sclerosis, MOGAD MOG antibody disease, ADEM acute disseminated encephalomyelitis, NMOSD neuromyelitis optica spectrum disorder, ON optic neuritis, CSF cerebrospinal fluid, IgM immunoglobulin M, IgG immunoglobulin G, AQP4 Aquaporin 4, LETM longitudinally extensive transverse myelitis, PLEX plasma exchange, MOG myelin oligodendrocyte glycoprotein, PCR polymerase chain reaction
Encephalitis/encephalomyelitis-like
A total of 71/78 patients (90%) presented by an encephalopathic clinical picture, lethargy, loss of consciousness and/or seizures which was supported by imaging findings. The median age of patients was 47 years. Hemorrhage and/or necrosis were reported in 26/71 (36.6%) of those patients. Of whom, 21/26 suffered severe COVID-19 infection which required mechanical ventilation. This observation raises the concern of a hypoxic ischemic theory and the need for anticoagulation in severely ill cases. A summary of the clinical characteristics is presented in Table 1.
MS-like demyelination
Three cases of MS-like demyelination: two females and one male have been reported. Their ages were 24, 28 and 29 years. The presentation was localizing to brain stem in two of them. On the other hand, unilateral optic neuritis was the main complaint in the third case.
In all cases the COVID-19 symptoms preceded the neurological symptoms by 2–4 weeks and the infection was mild in severity.
Oligoclonal bands were positive in two patients and not tested in the third.
Although the patient reported by Moore et al. fulfilled the 2017 McDonald criteria [12], we believe the MRI lesions were quite atypical being round in shape and larger than typical MS plaques. Follow-up is mandatory to securely establish the diagnosis.
The same atypical pattern of demyelination applies to the patient reported by Yavari et al. [5], where the lesions were large and located mainly in deep white matter and subcortical areas.
Despite the fact that the third case presented with optic neuritis, the associated field defect rendered the presentation atypical. Moreover, only two supratentorial lesions were detected in addition to the optic nerve lesion which does not fulfill the 2017 McDonald criteria.
That raises the question of whether these cases represent true MS or just a post-viral demyelinating syndrome. Findings of clinical characteristics are summarized in Table 2.
NMOSD and MOGAD-like demyelination
Longitudinally extensive transverse myelitis (LETM) and symptomatic cerebral syndrome are two of the well-known core features of NMOSD. In addition, ADEM could herald the first presentation of the disorder [13]. The patient reported by Zhogi et al. [14] was a 21-year-old male who presented with lethargy, vomiting, weakness of both lower limbs and urine retention, few weeks after suspicious COVID-19 symptoms.
Brain MRI revealed affection of bilateral corticospinal tracts up to cerebral peduncle and pons, marbled hyperintensity in the splenium of corpus callosum. Moreover, LETM was reported on his spinal MRI and testing for AQP4 antibody was positive. This patient received a probable diagnosis of COVID-19 based on his previous symptoms, chest imaging and positive antibody testing.
Additionally, Correa et al. [4] reported another female patient with LETM and brain hyperintensities in the fornix and subfornical area. The serology was positive for AQP4 after receiving a definite diagnosis of COVID-19 few weeks earlier.
MOGAD was reported in three post-COVID-19 patients based on positive antibody testing; the first was a 15-year-old patient who suffered subacute bilateral optic neuritis. COVID-19 infection was suspected based on his previous symptoms and two family members developing the infection a few weeks later. It was confirmed with positive nasopharyngeal PCR testing.
The second case was a 47-year-old male who presented with bilateral optic neuritis following asymptomatic infection, and was suspected based on his contact with positive cases. The infection was never confirmed though. Findings of clinical characteristics are shown in Table 2
The third case was a 4-year-old child presenting with ADEM. He had a mild infection with marked improvement after high-dose steroid treatment (Table 1).
Transverse myelitis
A total of 40 cases of TM have been reported in relation to SARS-CoV-2, of whom, 24 were isolated TM (Table 3), and 16 as a part of diffuse demyelinating process (Tables 1, 2). The cases included 19 females and 21 males.
Table 3.
Characteristics of cases presenting with COVID-19 related isolated myelitis
| Author | Age | Gender | Comorbidities | Time relation between infection and NP | Presenting COVID-19 symptoms | Presenting neurological symptoms | Neurological diagnosis | Other NS manifestations | Treatment | Response to treatment | Testing for SARS-COV-2 | Severity of COVID-19 infection | Antibody and OCBs testing | MRI data |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Valiuddin [64] | 61 | Female | None | A week | Rhinorrhea, chills and generalized weakness | Numbness and tingling in hands and feet, weakness in both lower limbs and upper limbs, constipation and urine retention |
Acute COVID myelitis (LETM) |
Acute Motor Axonal Neuropathy (AMAN) | IVMP for 5 days, 5 sessions PLEX |
No improvement Mild improvement |
Positive PCR in nasopharynx, negative in CSF | Mild | NA | Cervico-thoraco-lumbar spine: LETM without pathological contrast enhancement |
| 2. Moreno-Escobar [65] | 41 | Male | None | A week | Headache, nausea and low-grade fever, fatigue and myalgia |
Paresthesia of bilateral upper and lower extremities along with urinary and fecal retention Weakness of both lower limbs |
Post COVID-19 myelitis vs NMOSD (LETM) | Dysautonomia | IVMP for 5 days with oral taper | Partial | Positive PCR in nasopharynx | Mild | Negative OCBs, AQP4 and MOG antibodies | Cervical and thoracic spinal: LETM without any abnormal enhancement |
| 3. Munz et al. [66] | 60 | Male | HTN, fatty liver, ureterolithiasis | 3 days | Respiratory symptoms | Bladder dysfunction and progressive weakness of the lower limbs | Post-COVID myelitis | None | IV Acyclovir and ceftriaxone, IVMP 100 mg/day | Marked but partial | Positive PCR in nasopharynx, negative in CSF | Moderate | Negative OCBs |
Thoracic spinal: T2 signal hyperintensity of the thoracic spinal cord at Th9 level suggestive of acute transverse myelitis rather than multiple sclerosis FUP after 6 days: a patchy hyper- intensity of the thoracic cord at Th9-10 and at Th3-5 level, suggestive of transverse myelitis |
| 4. Sarma et al. [67] | 28 | Female | Hypothyroidism | At initial presentation | Productive cough, fever, myalgia, rhinorrhea | Low back pain, paresthesia in both lower limbs, urine retention, nausea and vomiting | Immune mediated COVID-myelitis (LETM) | None | Prednisolone and received two PLEX treatments | Partial | Positive PCR in nasopharynx | Mild | NA | Spine: widespread elongated signal changes throughout the spinal cord to the conus medullaris and involving the medulla (LETM) |
| 5. Sotoca et al. [68] | 69 | Female | None | 8 days | Fever and cough | Irradiated cervical pain, imbalance, and motor weakness and numbness in the left hand | Acute necrotizing myelitis (ANM) (LETM) | None | IVMP for 5 days, PLEX and another course of IVMP for 5 days with oral taper | Partial then deteriorated and new attack | Positive PCR in nasopharynx, negative in CSF | Mild | Negative OCBs, MOG and AQP4 antibodies |
Spinal: LETM extending from the medulla oblongata to C7, involving most of the cord with diffuse patchy enhancing lesions A new spinal MRI after deterioration: transversally and caudally progression until T6 level with similar enhancement and a new area of central necrosis at the T1 level with peripheral enhancement FUP MRI after PLEX: substantial decrease in myelitis extension and enhancement, but central necrosis at the C7-T1 level remained unchanged |
| 6. Domingues et al. [69] | 42 | Female | None |
3 weeks Symptoms overlapped |
Coryza, nasal obstruction | Recurrent paresthesia of the left upper limb, later progressing to left hemithorax, and hemiface (these symptoms occurred 3 years ago) | Spinal CIS vs viral myelitis | None | No treatment received | Full spontaneous recovery after 3 weeks | Positive PCR in CSF, negative in nasopharynx | Definite | NA | Cervical: small lateral demyelinating patch that explains the symptoms |
| 7. Alketbi et al. [70] | 32 | Male | None | 2 days | High-grade fever and flu-like symptoms | Sudden onset of bilateral lower limb weakness, difficulty in sitting up, and in passing urine | Post-COVID-19 myelitis (LETM) | None | IVMP for 5 days | Marked partial | Positive PCR in nasopharynx | Mild | NA | Spinal: LETM |
| 8. Durrani et al. [71] | 24 | Male | None | 12 days | Fever, chills, nausea and vomiting | Bilateral lower extremity weakness in addition to developing overflow urinary incontinence |
Post COVID- 19 myelitis vs COVID-19 myelitis (LETM) |
None | IVMP | Marked | Positive PCR in nasopharynx | Moderate | Negative OCBs | Spinal: LETM |
| 9. Abdelhady et al. [71] | 52 | Male | DM, G6PD deficiency | At initial presentation | Fever | Lower abdominal pain and inability to pass urine for the past 3 days, associated with fever and lower limb weakness | COVID-19 myelitis (LETM) | None | Steroids and acyclovir | Died | Positive PCR in nasopharynx, negative in CSF | Mild | NA | Thoracic spinal: LETM |
| 10. Chow et al. [72] | 60 | Male | HTN. Hypercholesterolemia, ex smoker | 18 days | Fever, cough, loss of taste and smell | Bilateral lower limb weakness, urinary retention and constipation | ATM (LETM) | None | IVMP for 3 days, physiotherapy | Complete neurological and radiological improvement | Positive PCR in nasopharynx | Mild | Negative serum anti-MOG, anti-MAG, and AQP4 antibodies |
Whole spine: LETM Normal MRI brain and orbit Follow-up MRI whole spine after 10 days showed almost complete resolution |
| 11. Kaur et al. [73] | 3 | Female | None | At initial presentation | Asymptomatic | Flaccid quadriparesis, neurogenic respiratory failure requiring intubation | ATM (LETM) | None | IVMP for 5 days (30 mg/kg/day) and IVIG (2 g/kg total dose), then seven sessions of PLEX, then Rituximab | No improvement | Positive PCR in nasopharynx | Mild | Negative serum AQP4 and MOG autoantibodies |
Spine: LETM of cervical spinal cord extending from the lower medulla to the mid-thoracic level with no enhancement Brain and orbits: were normal Follow-up MRI: reduced edema, early cervical myelomalacia |
| 12. Masuccio et al. [74] | 70 | Female | HTN, obesity | 15 days | Fever, anosmia and generalized myalgia | Severe quadriparesis, decreased tactile and pain sensation in the lower limbs accompanied with urinary retention | ATM | Acute motor axonal neuropathy (AMAN) | PLEX followed by one course of IVIG | No improvement | Negative PCR in nasopharynx | Mild | Anti-GD1b IgM positive | Spine: hyperintensity in posterior portion of the spinal cord from vertebral levels (C7–D1), no gadolinium enhancement |
| 13. Shahali et al. [75] | 63 | Male | DM, CRF, IHD | 4 days | Fever, fatigue, sore throat, and runny nose | Severe paraplegia, constipation, and urinary retention | ATM (LETM) | None | IVMP for 3 days and then tapered to 1 mg/kg/day), followed by IVIG (2.5 g daily for 3 days) | Complete resolution of neurologic manifestations | Positive PCR in nasopharynx | Moderate | IgG index = elevated (> 0.91) | Spine: LETM with linear enhancement within the mid and lower thoracic cord |
| 14. Chakraborty et al. [76] | 59 | Female | None | 4 days | Fever | Acute, severe progressive ascending flaccid paraplegia with retention of urine and constipation | ATM | None | IVMP at a dose of 1 g/day | Cardiac arrest, and death | Positive PCR in nasopharynx | Severe | NA | Thoracic spine: hyperintensity in the spinal cord at T6–T7 vertebral level, suggestive of myelitis |
| 15. Baghbanian et al. [77] | 53 | Female | DM, HTN, IHD | 14 days | Fever, respiratory symptoms | Paraplegia, low back pain and urinary incontinence | ATM (LETM) | None | PLEX | Partial recovery | Positive PCR in nasopharynx | Mild |
Negative CSF OCBs and the IgG index was in the upper limit of normal AQP4 and MOG antibodies were negative |
Spine: LETM in the T8–T10 cord segments Brain: normal |
| 16. Guler et al. [78] | 14 | Female | None | At initial presentation | Asymptomatic | Right hemiplegia | ATM (LETM) | None | IVIG was administered at 400 mg/kg/day for 5 days. Followed by IVMP was given at 30 mg/kg/day for 7 days | Partial improvement | Positive PCR in nasopharynx | Mild |
Serum AQP4 IgG was negative CSF OCBs were negative |
Spine: showed a contrast-enhancing lesion causing expansion at the C2–C5 level |
| 17. Fumery et al. [79] | 38 | Female | None | 14 days | Dry cough, myalgia, fatigue and shortness of breath | Paraplegia, hypoesthesia and bladder dysfunction | ATM (LETM) | None | IVMP (1 g daily for 8 consecutive days) | Significant clinical improvement | Positive PCR in nasopharynx | Mild |
Negative for OCBs and CSF SARS-CoV-2 PCR Negative MOG and AQP4 antibodies |
Spine: LETM with no gadolinium enhancement Brain: normal |
| 18. Nejad Bilgari et al. [80] | 11 | Female | None | 3 days | Fever | Flaccid paraplegia, urinary and fecal retention, epigastric pain | ATM (LETM) | None | IVIG (0.4 g/kg/day) for 5 days, IVMP (30 mg/kg) for 3 days, and seven sessions of PLEX | Slight improvement | Positive PCR in nasopharynx | Mild | NA |
Spinal: LETM Brain: normal |
| 19. Ali et al. [81] | 56 | Male | DM, G6PD deficiency | 3 days | Fever, fatigue, dyspnea | Flaccid paraplegia, urinary incontinence | ATM (LETM) | None | IV pulse steroids and acyclovir | No improvement, cardiac arrest, death | Positive PCR in nasopharynx | Moderate | NA |
Thoracic spine: LETM with no post-contrast enhancement Brain: normal |
| 20. Román et al. [82] | 72 | Male | HTN | None |
Asymptomatic Contact of a positive case |
Urine retention Dysesthesias in arms and legs and weakness of all four limbs |
ATM | None | IVMP (1 g/day) for 5 days, enoxaparin 40 mg daily, followed by IVIG (30 g/day) for five days. Oral prednisone was prescribed for the next 30 days | Partial improvement | Positive serology | Mild | Positive OCBs |
Brain: normal Cervicothoracic spinal: hyperintensities at C4–C5 and Th3–Th4 were observed without contrast enhancement |
| 21. Paterson et al. [3] | 48 | Male | DM, HTN | 19 days | Cough, dyspnea and fever | Numbness of hands and feet; band of itching sensation at level of the umbilicus and ataxia | Post-infectious myelitis (LETM) | None | IVMP for 3 days | Partial improvement | NA | Mild | Negative OCBs |
Brain: normal Thoracic spine: LETM down to the conus with no enhancement with contrast |
| 22. Saberi et al. [83] | 60 | Male | DM, HTN, hyperlipidemia | 2 weeks | Fever, nausea and vomiting | Progressive weakness of lower limbs accompanied by urinary incontinence and constipation | Post-infectious myelitis (LETM) | None |
IVIG (30 g/day) was initiated for 5 days PLEX for 5 days |
Improved initially then worsened again No improvement |
Negative PCR in nasopharynx | Mild | Negative AQP4 antibodies |
Cervical spine: LETM In the second cervical MRI, the previous hyperintense lesion was smaller and shrunken |
| 23. Lindan et al. [29] | 3 | Female | None | 1 day | Fever, diarrhea, urinary retention, hyperreflexia | Upper and lower extremity weakness, acute respiratory failures, confusion | Myelitis (LETM) | None | Supportive measures in ICU | No improvement | Positive PCR in nasopharynx | Severe | NA |
Brain: normal Spine: expansible T2-hyperintense signal from obex to mid-thoracic cord with mild enhancement Follow-up 4 days: worsening cord edema with extensive restricted diffusion, hemorrhage and enhancement Follow-up 3 weeks: interval myelomalacia with persistent restricted diffusion |
| 24. Lindan et al.29 | 12 | Male | None | 3 days | Fever, diarrhea | Urinary retention, hyperreflexia | Myelitis (LETM) | None | High-dose steroids | Partial improvement | Positive PCR in nasopharynx | NA | NA | Spine: long segment T2-hyperintensity from the obex through the mid-thoracic cord, with central predominance. No post-contrast imaging |
NP neurological presentation, NS nervous system, LETM longitudinally extensive transverse myelitis, PLEX plasma exchange, IVMP intravenous methyl prednisolone, IVIG intravenous immunoglobulin, OCBs oligoclonal bands, AQP4 Aquaporin4, MOG myelin oligodendrocyte glycoprotein, FUP follow-up, ANM acute necrotizing myelitis, CIS clinically isolated syndrome, PCR polymerase chain reaction, TM transverse myelitis, HTN hypertension, ATM acute transverse myelitis, DM diabetes mellitus, CRF chronic renal failure, IHD ischemic heart disease, G6PD glucose 6-phosphate dehydrogenase deficiency
LETM was the most frequently reported pattern of spinal involvement reported in 72.5% of cases of myelitis (19/24 of cases of isolated TM and 10/16 of cases of diffuse demyelination). Conus medullaris involvement was reported in 3 cases (3/40).
There are many mechanisms by which SARS-CoV-2 can induce myelitis: acute viral myelitis, post-COVID-19 immune-mediated myelitis, ischemic myelitis, part of an inflammatory demyelinating syndrome triggered by COVID-19 infection (ADEM, MS, NMOSD, and MOGAD). Table 3 summarizes the reported cases of isolated myelitis with COVID-19 infection.
Demyelination in special populations (children, pregnancy and puerperium)
Twenty pediatric, one pregnant and one postpartum cases of COVID-19-related brain demyelination have been reported. Although the pregnant and postpartum females had severe COVID-19 infection that required mechanical ventilation, they recovered almost completely with high doses of steroids. The presentation was of typical ADEM in one and diffuse leukoencephalitis with microbleeds in the other.
Among the pediatric cohort, there were 12 males and 8 females, with a median age of 9 years. Of them, 60% (12/20) suffered from severe COVID-19 infection. All patients presented with a picture of ADEM with (5/20) or without associated myelitis. In the five patients with myelitis, four showed LETM. The outcome was favorable (marked to complete recovery) in 13/20 patients. COVID-19-related isolated TM was reported in five pediatric patients (Table 3). Their median age was 11 years. LETM was the presenting imaging feature in all of them. Unfortunately, the outcome ranged between no to partial improvement.
Discussion
Several recent studies have evaluated the possible mechanisms of COVID-19-associated demyelination. Viral infection has demonstrated the ability to induce an inflammatory response, activating myelin-specific T cells, which can accelerate the development of early or delayed virus-induced demyelination [15]. Historically, SARS-CoV-1 and MERS-CoV, which are genetically similar to SARS-CoV-2, has been associated with central demyelination in literature [7].
Several experimental studies [16] revealed that murine coronavirus infection of susceptible mice has led to an inflammatory demyelination similar to MS, with coronavirus RNA sequences and its antigen detected in the demyelinating lesions. Furthermore, in one study [17], HCoV-229E viral RNA and HCoV-myelin cross-reactive T cell lines were predominantly detectable in the CNS of 36% of patients with MS, compared to none in patients with other neurological diseases and normal controls.
Although the exact mechanism of virus spread in the CNS has not been established, the two possible explanations are either hematogenous spread from systemic circulation to CNS or trans-neuronal spread through the olfactory pathway. In addition, the CNS can be potentially compromised through an ischemic–hypoxic insult resulting from severe respiratory affection or by latent immune-mediated mechanisms.
SARS-CoV-2 exhibits neurotropic and neuro-invasive properties and can cause direct neurological damage, through binding to angiotensin-converting enzyme-2 (ACE-2) receptors, whose expression is ubiquitous, including the CNS, or via blood circulation through Virchow Robin spaces [11]. Moreover, delayed CNS damage appears to be mediated by an undesired immune reaction following acute infection, leading to CNS demyelination [18].
Accumulated evidence showed that SARS-CoV-2 and several proinflammatory cytokines, including IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, TNF-α, and IFN-γ, can cross the blood–brain barrier, affecting macrophages, microglia, and astrocytes, which are the principal cells that mediate innate immunity in the CNS, thus creating a perfect cytokine storm for a proinflammatory state [10, 19]. IL-6 is an important proinflammatory mediator that can induce an immune response in the nervous system, and plays a crucial role in regulating the immune response in MS. In experimental autoimmune encephalomyelitis (EAE) model of MS, IL-6 aggravates clinical manifestations, neuroinflammation, and demyelination, principally by promoting pathogenic T helper (Th) 17 cell generation in the peripheral lymphoid organs [20, 21]. The levels of IL-6 were found to be correlated with the severity of COVID-19 symptoms, and this dysregulation can affect both innate and acquired immunity [1]. Furthermore, most COVID-19 patients exhibit increased circulating levels of IL-17 [22], which has a documented role in MS pathogenesis, mainly based on the data obtained from EAE model [23].
Moreover, autopsy data showed activation of astrocytes and microglia with infiltration of cytotoxic T cells, particularly in the brainstem, in COVID-19 patients [24]. In addition, Toll-like receptors (TLR), the main pattern recognition receptors expressed by CNS cells, have played a significant role in the pathogenesis of MS, and EAE model [25]. TLR are also believed to play a significant role in the pathogenesis of COVID‐19, mainly through recognition of viral particles, activation of the innate immune system, and secretion of pro‐inflammatory cytokines [26].
Another possible explanation could be the production of antibodies against myelin triggered by the virus. This para-infectious or post-infectious etiology is reported in several cases of post-SARS-CoV-2 Guillain–Barre syndrome. SARS-CoV-2 may play a role in triggering MS, similar to the documented role of Epstein–Barr virus [27]. These key aspects represent a maladaptive immune response to SARS-CoV-2 characterized by hyperactivity of innate immunity followed by immune dysregulation.
In view of this data, the reported cases in this review support a demyelinating aspect to SARS-CoV-2 infection. In the majority of cases, COVID-19 infection was confirmed by RT-PCR testing and in the remaining by adequate clinical, radiological and serological testing. Although causality cannot be adequately established, there is enough evidence to warrant further large-scale studies.
Brain demyelination was reported in a good number of cases; 78 patients, with encephalitis/encephalomyelitis presentation being the most common. The development of hemorrhage is potentially related to the severity of the respiratory involvement. Hypoxia as a result of such severe infection is a very likely contributing mechanism.
Although MS and other demyelinating diseases were reported, caution is a must when interpreting these findings, as in some cases the clinical presentation and MRI lesions are atypical. Further supporting laboratory investigations such as OCBs, VEP and spinal cord imaging will be needed to support the diagnosis. Despite the presence of MOG antibodies in some cases, MOGAD diagnosis should be made with caution, as acute bilateral optic neuritis and ADEM could be triggered by COVID-19 infection. A close follow-up is recommended to establish a solid diagnosis.
The fact the cytokines are involved to a great extent in COVID-19 pathogenesis could explain the trigger of MS and other common demyelinating disorders as they share the same pathogenic mechanisms. This might also explain the beneficial effects of steroids, plasma exchange (PLEX) and intravenous immunoglobulins (IVIg) in many of the reported cases.
It is noteworthy to mention the high frequency of LETM in the cases presenting with myelitis. Although it is shared by many diseases like NMOSD, idiopathic TM, and ADEM, SARS-CoV-2 myelitis should be added to the list of differential diagnosis of LETM.
With regard to the pediatric age group, and contrary to adult population, CNS demyelination appears to be less common, and usually associated with the development of multisystem inflammatory syndrome (MIS-C) [28]. Demyelinating disease etiologies can be difficult to stratify in children, as many of the initial presentations overlap among disease and syndromes; however, the most frequent type was post-infectious, immune-mediated ADEM-like presentation, followed by TM, and isolated splenial lesions [29]. Furthermore, the time of presentation, and the constantly negative PCR in CSF samples from affected patients, strongly suggests a post-infectious mechanism for the pathogenesis of CNS demyelination [30]. However, rare cases of acute, rather than post-infectious cerebellar ataxia have been recently reported in children with COVID-19 [31].
There are some limitations to the current systematic review. The main limitation is that it was based on small number of case reports and case series, despite extensive search of available literature, which hindered the ability to perform a meta-analysis. Moreover, although the selected reports provided relevant information, there was great heterogeneity regarding several aspects of the collected data. Furthermore, in few cases, nasopharyngeal RT-PCR testing was not performed, and in the majority of cases, CSF-PCR was negative, probably due to delayed presentation. Despite these shortcomings, the current review provides preliminary data on SARS-CoV-2-associated demyelinating diseases that can guide neurologists in dealing with such cases, and help future research.
Conclusion
This systematic review has shown an association between SARS‐CoV‐2 infection and the development of different types of CNS demyelination in literature, although causality cannot be made with absolute certainty. A probable para-infectious or post-infectious immune-mediated etiology might be implicated in patients with COVID-19. We are currently facing a dilemma of diagnosing common neurological disorders in the setting of this viral infection, raising the question of whether there is causality in this association, or just coincidence. The long-term prognosis of such cases is not clear, which may have implications regarding the use of disease-modifying therapies, or symptomatic treatments, in these patients. This relationship needs to be clarified in future research.
Author contributions
III: conceptualization (equal); data curation (equal); investigation (equal); methodology (equal); project administration (equal); validation (equal); supervision (equal); writing—original draft (equal); writing—review and editing (equal). SS: conceptualization (equal); data curation (equal); investigation (equal); methodology (equal); project administration (equal); validation (equal); supervision (equal); writing—original draft (equal); writing—review and editing (equal).
Funding
No funding received.
Declarations
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Ismail Ibrahim Ismail and Sara Salama contributed equally to this work.
References
- 1.Andalib S, Biller J, Di Napoli M, Moghimi N, McCullough LD, Rubinos CA, et al. Peripheral nervous system manifestations associated with COVID-19. Curr Neurol Neurosci Rep. 2021;21(3):9. doi: 10.1007/s11910-021-01102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montalvan V, Lee J, Bueso T, De Toledo J, Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020;194:105921. doi: 10.1016/j.clineuro.2020.105921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143(10):3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corrêa DG, de Souza Lima FC, da Cruz Bezerra D, Coutinho AC, Hygino da Cruz LC. COVID-19 associated with encephalomyeloradiculitis and positive anti-aquaporin-4 antibodies: cause or coincidence? Mult Scler. 2021;27(6):973–976. doi: 10.1177/1352458520949988. [DOI] [PubMed] [Google Scholar]
- 5.Yavari F, Raji S, Moradi F, Saeidi M. Demyelinating changes alike to multiple sclerosis: a case report of rare manifestations of COVID-19. In: Banerjee TK, editor. Case reports in neurological medicine. London: Hindawi; 2020. pp. 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahalakshmi AM, Ray B, Tuladhar S, Bhat A, Paneyala S, Patteswari D, et al. Does COVID-19 contribute to development of neurological disease? Immun Inflamm Dis. 2021;9(1):48–58. doi: 10.1002/iid3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202(3):415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ann Yeh E, Collins A, Cohen ME, Duffner PK, Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 2004;113(1):e73–e76. doi: 10.1542/peds.113.1.e73. [DOI] [PubMed] [Google Scholar]
- 9.Verstrepen K, Baisier L, De Cauwer H. Neurological manifestations of COVID-19, SARS and MERS. Acta Neurol Belg. 2020;120(5):1051–1060. doi: 10.1007/s13760-020-01412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dubé M, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12(1):14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore L, Ghannam M, Manousakis G. A first presentation of multiple sclerosis with concurrent COVID-19 infection. eNeurologicalSci. 2021;22:100299. doi: 10.1016/j.ensci.2020.100299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zoghi A, Ramezani M, Roozbeh M, Darazam IA, Sahraian MA. A case of possible atypical demyelinating event of the central nervous system following COVID-19. Mult Scler Relat Disord. 2020;44:102324. doi: 10.1016/j.msard.2020.102324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perlman S, Zhao J. Roles of regulatory T cells and IL-10 in virus-induced demyelination. J Neuroimmunol. 2017;15(308):6–11. doi: 10.1016/j.jneuroim.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray RS, Cai GY, Hoel K, Zhang JY, Soike KF, Cabirac GF. Coronavirus infects and causes demyelination in primate central nervous system. Virology. 1992;188(1):274–284. doi: 10.1016/0042-6822(92)90757-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talbot PJ, Boucher A, Duquette P, Gruslin E (2005) Coronaviruses and neuroantigens: myelin proteins, myelin genes. In: Lavi E, Constantinescu CS (eds) Experimental models of multiple sclerosis [Internet]. Springer US, Boston [cited 2021 Jul 1], pp 781–791. 10.1007/0-387-25518-4_43
- 18.Lima M, Siokas V, Aloizou A-M, Liampas I, Mentis A-FA, Tsouris Z, et al. Unraveling the possible routes of SARS-COV-2 invasion into the central nervous system. Curr Treat Options Neurol. 2020;22(11):37. doi: 10.1007/s11940-020-00647-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9(1):1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petkovic F, Castellano B. The role of interleukin-6 in central nervous system demyelination. Neural Regen Res. 2016;11(12):1922. doi: 10.4103/1673-5374.195273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janssens K, Slaets H, Hellings N. Immunomodulatory properties of the IL-6 cytokine family in multiple sclerosis. Ann N Y Acad Sci. 2015;1351:52–60. doi: 10.1111/nyas.12821. [DOI] [PubMed] [Google Scholar]
- 22.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kostic M, Dzopalic T, Zivanovic S, Zivkovic N, Cvetanovic A, Stojanovic I, et al. IL-17 and glutamate excitotoxicity in the pathogenesis of multiple sclerosis. Scand J Immunol. 2014;79(3):181–186. doi: 10.1111/sji.12147. [DOI] [PubMed] [Google Scholar]
- 24.Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19(11):919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Racke MK, Drew PD (2009) Toll-like receptors in multiple sclerosis. In: Kielian T (ed) Toll-like receptors: roles in infection and neuropathology [Internet]. Springer, Berlin [cited 2021 Jul 1], pp 155–168 (Current Topics in Microbiology and Immunology; vol 336). 10.1007/978-3-642-00549-7_9 [DOI] [PMC free article] [PubMed]
- 26.Khanmohammadi S, Rezaei N. Role of Toll-like receptors in the pathogenesis of COVID-19. J Med Virol. 2021;93(5):2735–2739. doi: 10.1002/jmv.26826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamel WA, Ibrahim Ismail I, Al-Hashel JY. Guillain–Barre syndrome following COVID-19 infection: first case report from Kuwait and review of the literature. Dubai Med J. 2021;18:1–5. [Google Scholar]
- 28.Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindan CE, Mankad K, Ram D, Kociolek LK, Silvera VM, Boddaert N, et al. Neuroimaging manifestations in children with SARS-CoV-2 infection: a multinational, multicentre collaborative study. Lancet Child Adolesc Health. 2021;5(3):167–177. doi: 10.1016/S2352-4642(20)30362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stafstrom CE, Jantzie LL. COVID-19: neurological considerations in neonates and children. Children (Basel) 2020;7(9):E133. doi: 10.3390/children7090133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sotgiu S, Uzzau S, Pippia A, Carta A, Antonucci R. Expanding the spectrum of acute cerebellitis due to SARS-Cov-2. Pediatr Neurol. 2021;121:1–2. doi: 10.1016/j.pediatrneurol.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zanin L, Saraceno G, Panciani PP, Renisi G, Signorini L, Migliorati K, et al. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. 2020;162(7):1491–1494. doi: 10.1007/s00701-020-04374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brun G, Hak J-F, Coze S, Kaphan E, Carvelli J, Girard N, et al. COVID-19—white matter and globus pallidum lesions: demyelination or small-vessel vasculitis? Neurol Neuroimmunol Neuroinflamm. 2020;7(4):e777. doi: 10.1212/NXI.0000000000000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alqwaifly M. Demyelinating solitary lesion of the central nervous system associated with COVID-19: a case report and literature review. Int J Adv Med. 2020;7(12):1884. doi: 10.18203/2349-3933.ijam20204998. [DOI] [Google Scholar]
- 35.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020;296(2):E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varadan B, Shankar A, Rajakumar A, Subramanian S, Sathya AC, Hakeem AR, et al. Acute hemorrhagic leukoencephalitis in a COVID-19 patient—a case report with literature review. Neuroradiology. 2021;63(5):653–661. doi: 10.1007/s00234-021-02667-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yong MH, Chan YFZ, Liu J, Sanamandra SK, Kheok SW, Lim KC, et al. A rare case of acute hemorrhagic leukoencephalitis in a COVID-19 patient. J Neurol Sci. 2020;416:117035. doi: 10.1016/j.jns.2020.117035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chalil A, Baker CS, Johnston RB, Just C, Debicki DB, Mayich MS, et al. Acute hemorrhagic encephalitis related to COVID-19. Neurol Clin Pract. 2021;11(2):e147–e151. doi: 10.1212/CPJ.0000000000000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karapanayiotides T, Geka E, Prassopoulos P, Koutroulou I, Kollaras P, Kiourtzieva E, et al. Concentric demyelination pattern in COVID-19-associated acute haemorrhagic leukoencephalitis: a lurking catastrophe? Brain. 2020;143(12):e100. doi: 10.1093/brain/awaa375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Handa R, Nanda S, Prasad A, Anand R, Zutshi D, Dass SK, et al. Covid-19-associated acute haemorrhagic leukoencephalomyelitis. Neurol Sci. 2020;41(11):3023–3026. doi: 10.1007/s10072-020-04703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghosh R, Dubey S, Finsterer J, Chatterjee S, Ray BK (2020) SARS-CoV-2-associated acute hemorrhagic, necrotizing encephalitis (AHNE) presenting with cognitive impairment in a 44-year-old woman without comorbidities: a case report. Am J Case Rep [Internet] [cited 2021 Jun 30]. https://www.amjcaserep.com/abstract/index/idArt/925641 [DOI] [PMC free article] [PubMed]
- 42.Haqiqi A, Samuels TL, Lamb FJ, Moharrum T, Myers AE. Acute haemorrhagic leukoencephalitis (Hurst disease) in severe COVID-19 infection. Brain Behav Immun Health. 2021;12:100208. doi: 10.1016/j.bbih.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mullaguri N, Sivakumar S, Battineni A, Anand S, Vanderwerf J (2021) COVID-19 related acute hemorrhagic necrotizing encephalitis: a report of two cases and literature review. Cureus [Internet] [cited 2021 Jun 30]. https://www.cureus.com/articles/50440-covid-19-related-acute-hemorrhagic-necrotizing-encephalitis-a-report-of-two-cases-and-literature-review [DOI] [PMC free article] [PubMed]
- 44.Radmanesh A, Derman A, Lui YW, Raz E, Loh JP, Hagiwara M, et al. COVID-19-associated diffuse leukoencephalopathy and microhemorrhages. Radiology. 2020;297(1):E223–E227. doi: 10.1148/radiol.2020202040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sachs JR, Gibbs KW, Swor DE, Sweeney AP, Williams DW, Burdette JH, et al. COVID-19-associated leukoencephalopathy. Radiology. 2020;296(3):E184–E185. doi: 10.1148/radiol.2020201753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McLendon LA, Rao CK, Da Hora CC, Islamovic F, Galan FN. Post-COVID-19 acute disseminated encephalomyelitis in a 17-month-old. Pediatrics. 2021;147(6):e2020049678. doi: 10.1542/peds.2020-049678. [DOI] [PubMed] [Google Scholar]
- 47.Scullen T, Keen J, Mathkour M, Dumont AS, Kahn L. Coronavirus 2019 (COVID-19)-associated encephalopathies and cerebrovascular disease: the New Orleans Experience. World Neurosurg. 2020;141:e437–e446. doi: 10.1016/j.wneu.2020.05.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Virhammar J, Kumlien E, Fällmar D, Frithiof R, Jackmann S, Sköld MK, et al. Acute necrotizing encephalopathy with SARS-CoV-2 RNA confirmed in cerebrospinal fluid. Neurology. 2020;95(10):445–449. doi: 10.1212/WNL.0000000000010250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dixon L, Varley J, Gontsarova A, Mallon D, Tona F, Muir D, et al. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e789. doi: 10.1212/NXI.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montes-Ramirez J, Aquino-Lopez E. COVID-19-associated diffuse leukoencephalopathy and cerebral microbleeds during puerperium. Int J Gynecol Obstet. 2020;151(3):466–467. doi: 10.1002/ijgo.13399. [DOI] [PubMed] [Google Scholar]
- 51.McCuddy M, Kelkar P, Zhao Y, Wicklund D. Acute demyelinating encephalomyelitis (ADEM) in COVID-19 infection: a case series. Neurol India. 2020;68(5):1192–1195. doi: 10.4103/0028-3886.299174. [DOI] [PubMed] [Google Scholar]
- 52.Assunção FB, Fragoso DC, Donoso Scoppetta TLP, Martins Maia AC. COVID-19-associated acute disseminated encephalomyelitis-like disease. AJNR Am J Neuroradiol. 2021;42(4):E21–E23. doi: 10.3174/ajnr.A6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parsons T, Banks S, Bae C, Gelber J, Alahmadi H, Tichauer M. COVID-19-associated acute disseminated encephalomyelitis (ADEM) J Neurol. 2020;267(10):2799–2802. doi: 10.1007/s00415-020-09951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Langley L, Zeicu C, Whitton L, Pauls M. Acute disseminated encephalomyelitis (ADEM) associated with COVID-19. BMJ Case Rep. 2020;13(12):e239597. doi: 10.1136/bcr-2020-239597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wong PF, Craik S, Newman P, Makan A, Srinivasan K, Crawford E, et al. Lessons of the month 1: a case of rhombencephalitis as a rare complication of acute COVID-19 infection. Clin Med. 2020;20(3):293–294. doi: 10.7861/clinmed.2020-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Novi G, Rossi T, Pedemonte E, Saitta L, Rolla C, Roccatagliata L, et al. Acute disseminated encephalomyelitis after SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e797. doi: 10.1212/NXI.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Demirci Otluoglu G, Yener U, Demir MK, Yilmaz B. Encephalomyelitis associated with Covid-19 infection: case report. Br J Neurosurg. 2020;7:1–3. doi: 10.1080/02688697.2020.1787342. [DOI] [PubMed] [Google Scholar]
- 58.Utukuri PS, Bautista A, Lignelli A, Moonis G. Possible acute disseminated encephalomyelitis related to severe acute respiratory syndrome coronavirus 2 infection. AJNR Am J Neuroradiol. 2020;41:E82–E83. doi: 10.3174/ajnr.A6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lopes CCB, Brucki SMD, Passos Neto CEB, Corazza LA, Baima JPS, Fiorentino MD, et al. Acute disseminated encephalomyelitis in COVID-19: presentation of two cases and review of the literature. Arq Neuro-Psiquiatr. 2020;78(12):805–810. doi: 10.1590/0004-282x20200186. [DOI] [PubMed] [Google Scholar]
- 60.Kim HJ, Paul F, Lana-Peixoto MA, Tenembaum S, Asgari N, Palace J, et al. MRI characteristics of neuromyelitis optica spectrum disorder: an international update. Neurology. 2015;84(11):1165–1173. doi: 10.1212/WNL.0000000000001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Ruijter NS, Kramer G, Gons RAR, Hengstman GJD. Neuromyelitis optica spectrum disorder after presumed coronavirus (COVID-19) infection: a case report. Mult Scler Relat Disord. 2020;46:102474. doi: 10.1016/j.msard.2020.102474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou S, Jones-Lopez EC, Soneji DJ, Azevedo CJ, Patel VR. Myelin oligodendrocyte glycoprotein antibody-associated optic neuritis and myelitis in COVID-19. J Neuroophthalmol. 2020;40(3):398–402. doi: 10.1097/WNO.0000000000001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kogure C, Kikushima W, Fukuda Y, Hasebe Y, Takahashi T, Shibuya T, et al. Myelin oligodendrocyte glycoprotein antibody-associated optic neuritis in a COVID-19 patient: a case report. Medicine. 2021;100(19):e25865. doi: 10.1097/MD.0000000000025865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Valiuddin H, Skwirsk B, Paz-Arabo P. Acute transverse myelitis associated with SARS-CoV-2: a case-report. Brain Behav Immun Health. 2020;5:100091. doi: 10.1016/j.bbih.2020.100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moreno-Escobar MC, Kataria S, Khan E, Subedi R, Tandon M, Peshwe K, et al. Acute transverse myelitis with dysautonomia following SARS-CoV-2 infection: a case report and review of literature. J Neuroimmunol. 2021;353:577523. doi: 10.1016/j.jneuroim.2021.577523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Munz M, Wessendorf S, Koretsis G, Tewald F, Baegi R, Krämer S, et al. Acute transverse myelitis after COVID-19 pneumonia. J Neurol. 2020;267(8):2196–2197. doi: 10.1007/s00415-020-09934-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sarma D, Bilello L. A case report of acute transverse myelitis following novel coronavirus infection. Clin Pract Cases Emerg Med. 2020;4(3):321–323. doi: 10.5811/cpcem.2020.5.47937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sotoca J, Rodríguez-Álvarez Y. COVID-19-associated acute necrotizing myelitis. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e803. doi: 10.1212/NXI.0000000000000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Domingues RB, Mendes-Correa MC, de Moura Leite FBV, Sabino EC, Salarini DZ, Claro I, et al. First case of SARS-COV-2 sequencing in cerebrospinal fluid of a patient with suspected demyelinating disease. J Neurol. 2020;267(11):3154–3156. doi: 10.1007/s00415-020-09996-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.AlKetbi R, AlNuaimi D, AlMulla M, AlTalai N, Samir M, Kumar N, et al. Acute myelitis as a neurological complication of Covid-19: a case report and MRI findings. Radiol Case Rep. 2020;15(9):1591–1595. doi: 10.1016/j.radcr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Durrani M, Kucharski K, Smith Z, Fien S. Acute transverse myelitis secondary to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): a case report. Clin Pract Cases Emerg Med. 2020;4(3):344–348. doi: 10.5811/cpcem.2020.6.48462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chow CCN, Magnussen J, Ip J, Su Y. Acute transverse myelitis in COVID-19 infection. BMJ Case Rep. 2020;13(8):e236720. doi: 10.1136/bcr-2020-236720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaur H, Mason JA, Bajracharya M, McGee J, Gunderson MD, Hart BL, et al. Transverse myelitis in a child with COVID-19. Pediatr Neurol. 2020;112:5–6. doi: 10.1016/j.pediatrneurol.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Masuccio FG, Barra M, Claudio G, Claudio S. A rare case of acute motor axonal neuropathy and myelitis related to SARS-CoV-2 infection. J Neurol. 2021;268(7):2327–2330. doi: 10.1007/s00415-020-10219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shahali H, Ghasemi A, Farahani RH, Nezami Asl A, Hazrati E. Acute transverse myelitis after SARS-CoV-2 infection: a rare complicated case of rapid onset paraplegia. J Neurovirol. 2021;27(2):354–358. doi: 10.1007/s13365-021-00957-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chakraborty U, Chandra A, Ray AK, Biswas P. COVID-19-associated acute transverse myelitis: a rare entity. BMJ Case Rep. 2020;13(8):e238668. doi: 10.1136/bcr-2020-238668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baghbanian SM, Namazi F (2020) Post COVID-19 longitudinally extensive transverse myelitis (LETM)–a case report. Acta Neurol Belg [Internet] [cited 2021 Jul 1]. 10.1007/s13760-020-01497-x [DOI] [PMC free article] [PubMed]
- 78.Guler MA, Keskin F, Tan H. Acute myelitis secondary to COVID-19 in an adolescent: causality or coincidence? New Trend Med Sci. 2020;1(2):132–136. [Google Scholar]
- 79.Fumery T, Baudar C, Ossemann M, London F. Longitudinally extensive transverse myelitis following acute COVID-19 infection. Mult Scler Relat Disord. 2021;48:102723. doi: 10.1016/j.msard.2020.102723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nejad Biglari H, Sinaei R, Pezeshki S, Khajeh HF. Acute transverse myelitis of childhood due to novel coronavirus disease 2019: The first pediatric case report and review of literature. Iran J Child Neurol. 2021;15(1):107–112. doi: 10.22037/ijcn.v15i1.31579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ali L, Khan A, Elalamy O, Canibano B, Al hatou M, Adeli G, Ahmed I, Iqrar A, Abdussalam A, Ibrahim AS, Sardar S (2020) A rare presentation of acute flaccid myelitis in COVID-19 patient: a case report. Pak J Neurol Sci (PJNS) 15(3), Article 5
- 82.Román GC, Gracia F, Torres A, Palacios A, Gracia K, Harris D. Acute transverse myelitis (ATM): clinical review of 43 patients with COVID-19-associated ATM and 3 post-vaccination ATM serious adverse events with the ChAdOx1 nCoV-19 vaccine (AZD1222) Front Immunol. 2021;12:653786. doi: 10.3389/fimmu.2021.653786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saberi A, Ghayeghran A, Hatamian H, et al. COVID-19-associated myelitis, para/post infectious or infectious myelitis: a case report from the north of Iran. Caspian J Neurol Sci. 2020;6(2):132–138. doi: 10.32598/CJNS.6.21.1. [DOI] [Google Scholar]