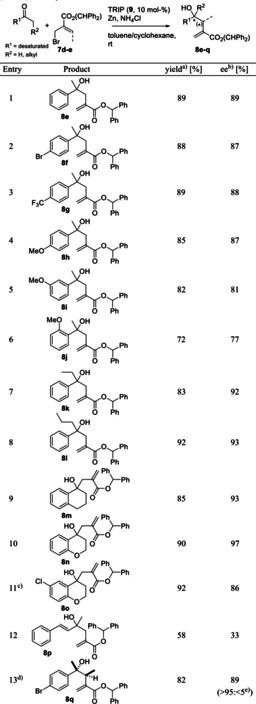
Table 3.
TRIP‐catalyzed allylation of different ketones under optimized conditions.
|
|
Reaction conditions: Ketone (0.1 mmol), zinc (5 eq.), NH4Cl (8 eq.), (S)‐TRIP [(S)‐9, 10 mol‐%], 7 d (1.5 eq.), 0.5–2 mL toluene/cyclohexane mixture (for details see ESI); stirred at rt for 16 h.
[a] Isolated yields are given.
[b] The enantiomeric excess was determined on a chiral stationary phase via normal phase HPLC‐UV.
[c] (R)‐TRIP [(R)‐9, 10 mol‐%] was used.
[d] Benzhydryl (Z)‐2‐(bromomethyl)but‐2‐enoate (7 e) was used as allylating reagent.
[e] The diastereomeric ratio (d.r.) was determined via 1H‐NMR (given in brackets); relative stereoconfiguration was determined by 1H,1H‐NOESY spectra of lactone 10 q (see Scheme 3 and the ESI).