FIGURE 1.
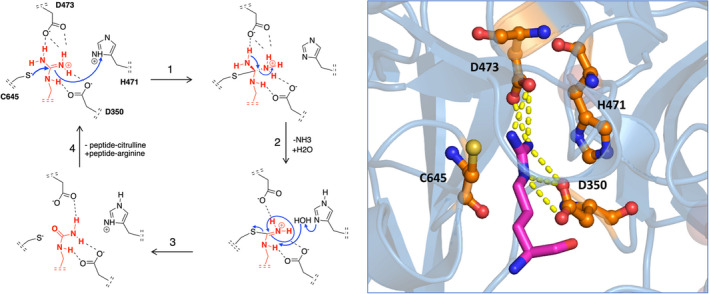
Chemical modification of arginine to citrulline by PAD4. The mechanism of citrullination by PAD4, as adapted from Knuckley et al.2 The PAD4 residues Asp350 and Asp473 function as “anchor points” and sit at the bottom of the tunnel, forming strong interactions via two salt bridges with the guanidinium group of the arginine substrate. The actual catalytic process of deimination is initiated by nucleophilic attack of thiolate‐form Cys645 on the guanidinium carbon of arginine, followed by stabilization of the tetrahedral intermediate through protonation by His471 (1). Subsequent collapse of the intermediate (2) yields free ammonia and an S‐alkylthiouronium intermediate. This intermediate is then hydrolyzed by a water molecule yielding the original Cys645 thiolate and citrulline (3). Release of the peptide‐citrulline product finishes the catalytic cycle (4). On the right, the crystal structure of PAD4 together with the substrate arginine is shown. The PAD4 active site resides are shown in orange, arginine is shown in magenta. Yellow dotted lines represent hydrogen bonds between arginine and aspartic acids D350 and D473. PAD, peptidyl arginine deiminase
