FIGURE 3.
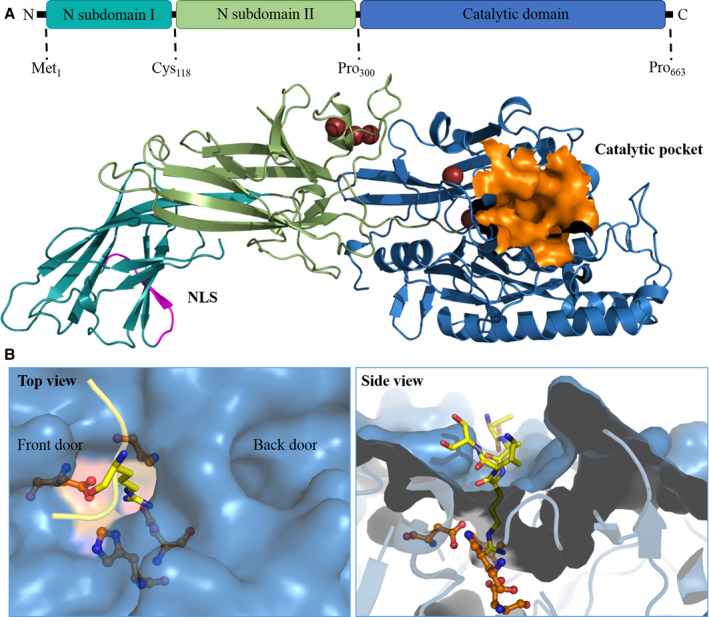
The three‐dimensional structure of PAD4. A, Full‐length PAD4 monomer. The homology model of PAD4 is made using YASARA software based on the 3B1U PDB template. The NLS is shown in magenta, and the catalytic pocket is shown in orange. B, The catalytic site and substrate‐binding pocket of PAD4. The arginine substrate containing histone tail (TARKS) is shown in yellow; active site residues are orange. The left panel shows the top view, where the TAKS residues of the histone tails are not shown. The front door and back door of the active site are indicated in the image. The right panel contains the side view of the binding pocket, where it is clear how only the substrate arginine protrudes into the active site. PAD, peptidyl arginine deiminase
