FIGURE 2.
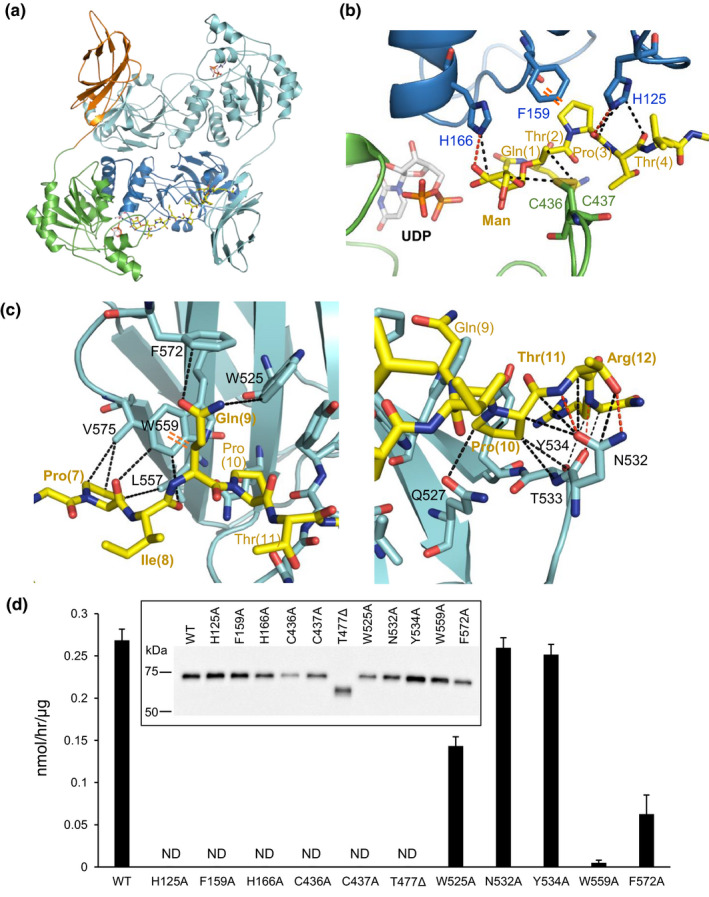
Crystal structure of sPOMGNT2 with mannosyl peptide. (a) Overall structure of the sPOMGNT2‐mannosyl peptide complex. Two UDP (gray and orange) and a 379Man short peptide (yellow) which are shown by stick models are in the sPOMGNT2 dimer. The different domains of one sPOMGNT2 molecule are colored as described in Figure 1b, and the other sPOMGNT2 molecule is colored pale blue. (b) Interaction between sPOMGNT2 and the 379Man short peptide in the region around mannose. The N‐lobe and C‐lobe of sPOMGNT2, mannosyl peptide, and UDP are shown by blue, green, yellow, and gray/orange color, respectively. The red and black dotted lines and an orange double dotted line are hydrogen bonds, hydrophobic interactions, and a CH‐π interaction, respectively. (c) Interaction between sPOMGNT2 and the C‐terminal region of the 379Man short peptide. The FnIII domain of another molecule of sPOMGNT2 (pale blue) different from that shown in panel B interacts with the C‐terminal region of the peptide (yellow). The types of interactions are shown in the same way as in panel (b). (d) Enzymatic activities of sPOMGNT2 mutants at which residues interact with mannosyl peptide. T477∆ is a deletion mutant which lacks the whole C‐terminal FnIII domain. sPOMGNT2 immunoprecipitated from the culture media was used. Inset: immunoblot analysis of sPOMGNT2 proteins to normalize input sPOMGNT2. ND means that the activities were not detected. Average values ± SE of three independent experiments are shown
