Figure 2:
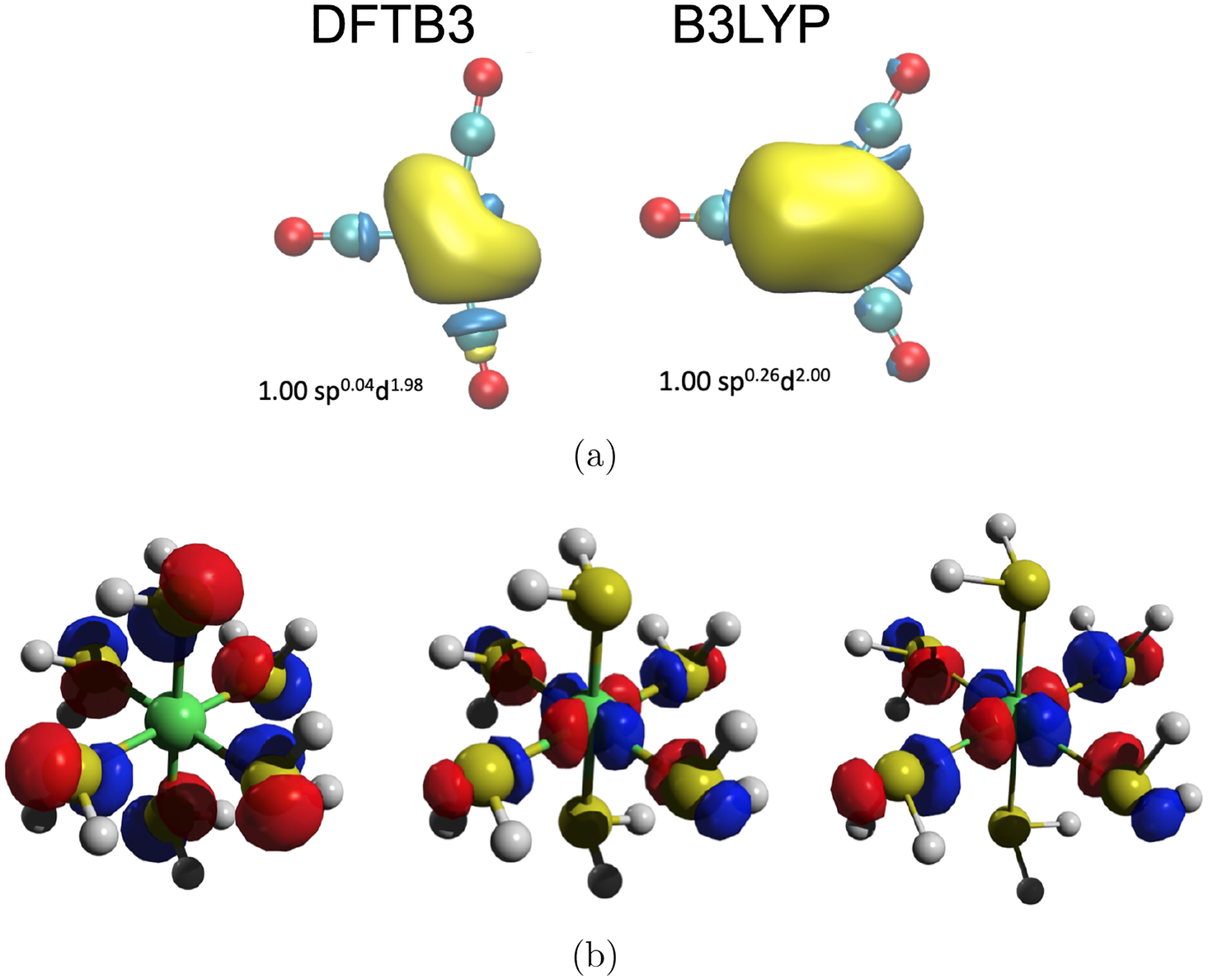
Description of Ni compounds with DFTB3. (a) Comparison of the nickel lone valence hybrid in [Ni(CO)3]2+ with DFTB3/3OB and B3LYP based Natural Bonding Orbital analyses;57,58 DFTB3 and B3LYP favor D3h and C2v symmetry, respectively. (b) Examples of frontier orbital comparisons between DFTB3, DFTB3+U and PBE calculations for high-spin [Ni(H2S)6]2+. Including the +U correction in DFTB3/3OB62 improves various properties such as d orbital populations, nature of frontier orbitals, ligand field splitting and energy difference between low/high-spin states.
