Figure 4:
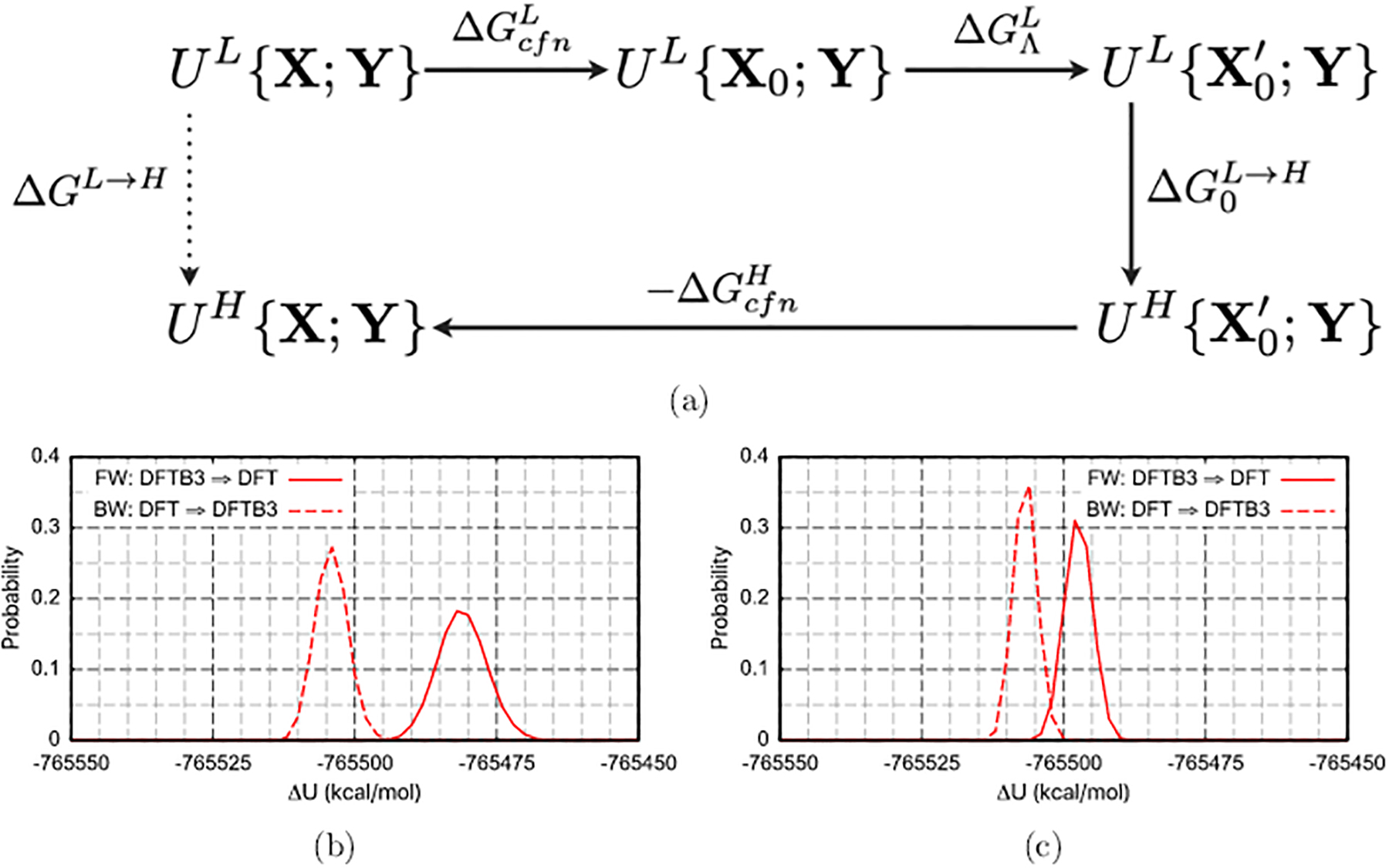
A staged transformation approach141 for computing the free energy difference at two levels (L/H) of theory, ΔGL→H. (A) The staged thermodynamic path treats selected degrees of freedom (X) separately from the rest (Y); X represents the degrees of freedom that lead to a large gap in the ΔULH distribution. Assuming that the free energy costs for confining X to values at (or near) the free energy minima are similar at the L and H levels, ΔGL→H is given by the sum of , which converges readily since the sampling involves only Y, and the “reorganization free energy”, , which is the free energy cost of changing X0 to at the low level of theory. (b-c) Illustration of the impact of bond and angle restraints on the ΔULH distribution for a methyl diphosphate, which is treated with either DFTB3 (L) or B3LYP (H), solvated by TIP3P water.
