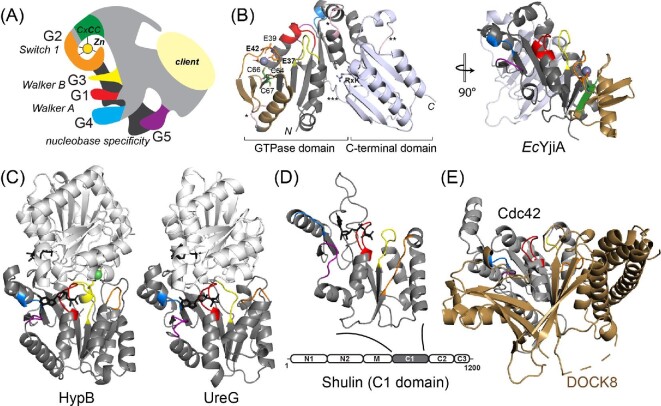
Fig. 3.
COG0523 structural models. (A) ‘Elephant’ representation of COG0523 metallochaperones with G-loops and nucleotide binding and hydrolysis motifs highlighted.1 The variable C-terminal domain is thought to harbor sequence-specific determinants for specific interactions with a client protein(s). (B) The only metal-bound COG0523 structure, of E. coli YjiA (PDB: 4ixm). The GTPase and C-terminal domains are indicated and the G-loops are shaded as in panel A. The coordination structure of the high affinity, regulatory ZnII site is shown, proposed to involve E37, E42, and C66; the side chain of E39, also highlighted, appears to point away from the metal, but could not be modeled past Cβ.29 This side chain also points away from the metal binding cleft in the apo-YjiA structure.63 This metal site localizes to the G2 loop and the β-meander63 (shaded gold) defines an insertion in a canonical G3E P-loop GTPase fold (right). (C) Ribbon structures of Helicobacter pylori HypB (4lps)24 and H. pylori UreG (4hi0)112 dimers (one subunit shaded gray, the other white) with the G-loops in the gray protomer shaded as in panels A and B in the same orientation as EcYjiA, (panel B, right). GTP analog is shown in black stick. (D) Structure of Tetrahymena Shulin C1 domain illustrating structural similarity to G3E P-loop GTPases;81 however, sequence similarity to EcYjiA is undetectable. (E) Ribbon representation of the vertebrate Cdc42-DOCK8 complex (3vh1), with Cdc42 shaded gray and G-loops colored as above, and DOCK8, a Cdc42-specific guanine nucleotide exchange factor, (GEF) shaded gold.65