Abstract
Background
Midlife cardiovascular risk factors (CVRFs) increase risk of dementia. Black Americans experience an elevated prevalence of CVRFs and dementia. However, little is known of how CVRFs prior to midlife affect late-life cognition. We examined CVRFs in adolescence, young adulthood, and midlife with late-life cognition in the Study of Healthy Aging in African Americans (STAR).
Method
STAR assesses cognitive aging among 764 Black Americans aged ≥50 (mean age = 69; SD = 9; range = 53–95). Participants’ body mass index, blood pressure, glucose, and total cholesterol were collected during Multiphasic Health Checkups (MHC; 1964–1985). At STAR baseline (2018–2019), executive function, verbal episodic memory, and semantic memory were measured using the Spanish and English Neuropsychological Assessment Scales. Linear regression models examined associations between CVRFs and cognition adjusting for demographics and years since MHC.
Results
At MHC, 36% of participants had 1 CVRF and 26% had ≥2. Twenty-two percent of participants were adolescents (age 12–20), 62% young adults (age 21–34), and 16% midlife adults (age 35–56). Overweight/obesity was not associated with cognition. Hypertension was associated with worse executive function (β [95% CI]: −0.14 [−0.28, −0.0003]) and verbal episodic memory (β [95% CI]: −0.22 [−0.37, −0.07]) compared to normotension. Diabetes was associated with worse executive function (β [95% CI]: −0.43 [−0.83, −0.03]). Having ≥2 CVRFs (vs 0) was associated with worse executive function (β [95% CI]: −0.19 [−0.34, −0.03]) and verbal episodic memory (β [95% CI]: −0.25 [−0.41, −0.08]). Adolescents with hypertension had lower late-life executive function compared to normotensive adolescents (β [95% CI]: −0.39 [−0.67, −0.11]). Young adulthood hypertension (β [95% CI]: −0.29 [−0.49, −0.09]) and midlife hyperlipidemia (β [95% CI]: −0.386 [−0.70, −0.02]) were associated with lower verbal episodic memory.
Conclusions
Among Black Americans, life-course CVRFs were associated with poorer executive function and verbal episodic memory emphasizing the importance of cardiovascular health on the aging brain.
Keywords: Black Americans, Cardiovascular disease, Cognitive aging, Dementia, Life course
Black Americans experience a disproportionate burden of cardiovascular risk factors (CVRFs) including overweight/obesity, hypertension, diabetes, and hyperlipidemia (1,2). The National Health and Nutrition Examination Survey (NHANES) estimated age-adjusted prevalence of 48% for obesity, 42% for hypertension, 20% for diabetes, and 10% for high total cholesterol among Black Americans aged 20 and older (2). However, due to the long and ongoing history of racism against Black Americans, they are also more likely to experience discrimination and low socioeconomic status across the life course (3). These psychosocial factors are associated with inflammatory biomarkers that, over time, have deleterious effects on health in a process called weathering (4). Weathering is associated with overweight/obesity, hypertension, hyperlipidemia, and diabetes (4) and may make Black Americans more vulnerable to the chronic conditions associated with CVRFs.
CVRFs have been linked to a number of poor health outcomes including cardiovascular disease (CVD), stroke, and CVD mortality (5–10) and are increasingly recognized as risk factors for poor brain health including lower cognitive function, cognitive decline, and dementia (11–17). Several studies have noted that Black Americans have worse late-life cognitive function and higher risk of cognitive impairment compared to other racial/ethnic groups (18–20). However, it remains unclear whether high rates of cognitive impairment in this population are driven by the disproportionate burden of CVRFs. The majority of studies linking cardiovascular and brain health have been conducted in predominantly White, middle-aged cohorts (21). Additionally, little is known about the impact of life-course timing of risk factors on cognition, particularly when risk factors develop prior to midlife (22–24). The disparate burden of CVRFs and dementia in Black Americans needs further examination from a life-course perspective.
Using data from the Study of Healthy Aging in African Americans (STAR), our aim was to characterize the relationship between CVRFs measured in adolescence, young adulthood, and midlife with late-life cognition. We hypothesized that prevalence of CVRFs would be highest in midlife, followed by young adulthood and adolescence. Further, we hypothesized that CVRFs during adolescence, young adulthood, and midlife would be associated with worse late-life cognition and that having multiple CVRFs would have a cumulative, negative effect on cognitive function.
Method
STAR includes community-dwelling older Black adults living in the San Francisco Bay area of California, primarily the cities of Oakland and Richmond. The objective of STAR is to evaluate how life-course vascular and sociocultural factors influence the trajectory of cognitive aging and burden of cognitive impairment among Black Americans. Individuals eligible for study participation were long-term members of an integrated health care delivery system, Kaiser Permanente Northern California (KPNC). Participants had to identify as Black American, be aged 50 years or older by January 1, 2018, and have previously participated in Kaiser Permanente Multiphasic Health Checkup (MHC) exams between 1964 and 1985. In order to recruit approximately equal proportions of participants aged 50–64 and 65 and older, stratified random sampling by age and educational attainment was used (age range: 53–95 years). Individuals were excluded from participation if they had an electronic medical record diagnosis of dementia or other neurodegenerative diseases (eg, frontotemporal dementia, Lewy body disease, Parkinson’s disease with dementia) or health conditions that would hinder participation in study interviews (eg, hospice activity in the last year, history of end-stage renal disease or dialysis in the last year, history of severe chronic obstructive pulmonary disease in the last 6 months, or congestive heart failure hospitalizations in the last 6 months). This study was conducted with IRB approval (#1121043-6), and all participants provided written, informed consent.
MHC exams were conducted as part of routine care at KPNC and administered in 5 waves from 1964 to 1973, 1973 to 1977, 1977 to 1985, 1985 to 1992, and 1992 to 1996. Participants could attend multiple MHCs; however, only data from each participant’s first MHC were available for this analysis. As such, all first visits fell during the first 3 MHC waves (1964–1985). Data collection at MHC included blood and urine samples, physical measures (such as weight and height), and questionnaires about lifestyle, health behaviors, and health history. These data were used to ascertain CVRFs. Participants were put into MHC age groups based on their first MHC exam age: adolescents (age 12–20), young adults (age 21–34), and midlife adults (age 35–56). Risk factors of hypertension (defined as a systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, self-report of high blood pressure diagnosis, or self-report of antihypertensive medication use), overweight/obesity (defined as a body mass index of ≥25 kg/m2), hyperlipidemia (defined as a fasting serum total cholesterol ≥200 mg/dL), and diabetes (defined as a fasting serum glucose ≥126 mg/dL or a nonfasting serum glucose of ≥ 200, self-report of a diabetes diagnosis, or self-report of insulin medication use) were measured by clinical staff using standard methods and dichotomized (yes/no). We could not differentiate between cases of type 1 and type 2 diabetes, and it is likely we captured cases of both. We also created a categorical risk factor variable based on the number of risk factors each participant had at their first MHC with categories of 0 risk factors, 1 risk factor, or 2+ risk factors.
Cognitive function was measured using the Spanish and English Neuropsychological Assessment Scales (SENAS). The SENAS is a battery of cognitive tests that was developed using item response theory (IRT) methodology and has been extensively validated for measuring and comparing cognitive change across diverse racial/ethnic groups and English and Spanish language administrations (25–32). It has been used as the primary cognitive outcome in many studies examining how late-life cognitive decline in diverse older adults is associated with life-course risk and protective factors (33,34), change in clinical status (27), neuroimaging (35–37), and cognitive reserve (38–42). SENAS administration procedures, development, and psychometric characteristics are described in detail elsewhere (26,29). All STAR participants completed the SENAS in English. An executive function composite score was calculated from IRT-based component measures of category fluency, phonemic (letter) fluency, and working memory (digit-span backward, visual-span backward, list sorting). Verbal episodic memory composite scores were derived using an IRT-based multitrial word-list learning test, and a semantic memory composite score was calculated from IRT-based verbal (object-naming) and nonverbal (picture association) tests. These scores do not have floor or ceiling affects and are normally distributed (26). Cognitive test scores for each domain were z-standardized using the mean and standard deviation from the full baseline sample.
Covariates included age at STAR interview (from participants’ medical records), years since first MHC exam (estimated by subtracting age at MHC from age at STAR interview), gender (male/female), educational attainment, and parental education. At STAR Wave 1, participants were asked about their gender with the following response options: male, female, other (with space to write in gender identity), or refused. For participants with missing gender data, gender was ascertained from the medical record and likely based on sex. Education was self-reported at Wave 1 as the last or highest level of school completed for credit and then dichotomized as some college or less education or college graduate or more education for analyses. Parental education was ascertained by asking participants for the highest educational level of their mother, father, or the person that raised them. Responses were dichotomized as less than high school or high school graduate or more. If educational attainment of 2 parents/guardians was provided, parental education was determined by the highest level of education reported between the 2. These covariates were chosen as possible confounders on the relationship between CVRFs and cognition.
Statistical Analysis
Of 764 participants enrolled in STAR, 9 were excluded from the analyses due to missing age at MHC (n = 4), any of the 3 cognitive domain scores (n = 5), or data on all 4 CVRFs (n = 0) for a final analytic sample of 755 participants. We provided means and prevalence of baseline characteristics and CVRFs stratified by MHC age group. We used linear regression to assess the associations of overweight/obesity, hypertension, hyperlipidemia, and diabetes with late-life cognitive domain z-scores. Each risk factor was tested separately and participants without the risk factor of interest were used as the reference. Linear regression was also used to assess the relationship between number of risk factors with domain-specific late-life cognition using those with no CVRF as the reference. Models were run in 3 stages, all of which adjusted for age at STAR Wave 1 interview, years since first MHC, gender, education, and parental education. We first ran overall models, followed by overall models testing for a CVRF × Age group interaction, and models stratified by MHC age group. We explored stratified results regardless of whether interaction terms were significant, because small sample sizes limited our ability to detect statistically significant interactions, but we wanted to examine exploratory trends. The association between diabetes and cognition stratified by age group was not assessed due to the small number of cases. Analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
At MHC, 22% (n = 166) of participants were adolescents (age 12–20; mean age = 18, SD = 2), 62% (n = 468) of participants were young adults (age 21–34; mean age = 27, SD = 4), and 16% (n = 121) of participants were midlife adults (age 35–56; mean age = 39, SD = 4). Overall, the mean age of participants at MHC was 27 (SD = 7) years, and the mean age at STAR was 69 (SD = 9) years. An average of 42 (SD = 5) years elapsed between CVRF measurement at MHC and cognitive assessment at STAR. Participants who were categorized as midlife adults at MHC were more likely to be male (37%), more likely to have not graduated college (72%), and more likely to have 2 or more CVRFs at MHC (48%) compared to younger age groups (Table 1; Figure 1). Participants who were adolescents at MHC were the less likely to be male (25%), less likely to have not graduated college (63%), and the least likely to have 2 or more CVRFs at MHC (14%) compared to older age groups. Young adults at MHC fell between adults and adolescents in the proportion that were male (33%), did not graduate college (64%), and had 2 or more CVRFs (24%).
Table 1.
STAR Participant Baseline Characteristics Stratified by Age Group at Multiphasic Health Checkups
| Variables | Overall | Adolescence (age 12–20) | Young Adulthood (age 21–34) | Midlife Adulthood (age 35–56) |
|---|---|---|---|---|
| n = 755 | n = 166 | n = 468 | n = 121 | |
| MHCa (1964–1985) | ||||
| Age at MHC, y | 26.8 ± 7.3 | 18.2 ± 1.5 | 26.6 ± 3.8 | 39.3 ± 4.1 |
| Overweight/obeseb, % (n) | 33.2 (251) | 22.9 (38) | 32.5 (152) | 50.4 (61) |
| Hypertensionc, % (n) | 20.7 (156) | 19.6 (32) | 19.2 (90) | 28.1 (34) |
| Hyperlipidemiad, % (n) | 36.5 (269) | 21.8 (36) | 36.4 (165) | 56.7 (68) |
| Diabetese, % (n) | 2.1 (16) | 1.2 (2) | 1.5 (7) | 5.8 (7) |
| STAR baseline (2018–2019) | ||||
| Age at STAR, y | 68.7± 8.8 | 60.9 ± 4.2 | 68.5 ± 7.3 | 80.2 ± 6.0 |
| Time between MHC and STAR, y | 41.9 ± 5.4 | 42.7 ± 4.7 | 41.9 ± 5.5 | 41.0 ± 5.7 |
| Male gender, % (n) | 31.7 (239) | 25.3 (42) | 32.5 (152) | 37.2 (45) |
| Participant education | ||||
| College graduate, % (n) | 34.6 (260) | 37.0 (61) | 35.6 (166) | 27.5 (33) |
| Some college or vocational/trade school, % (n) | 34.6 (260) | 37.0 (61) | 35.6 (166) | 27.5 (33) |
| High school graduate or less, % (n) | 34.6 (260) | 37.0 (61) | 35.6 (166) | 27.5 (33) |
| Parental education | ||||
| High school graduate or less, % (n) | 40.0 (304) | 31.0 (52) | 43.1 (202) | 41.0 (50) |
Notes: MHC = Multiphasic Health Checkups; STAR = Study of Healthy Aging in African Americans.
aMHC conducted as part of routine care between 1964 and 1985.
bOverweight defined as body mass index (BMI) of ≥25 kg/m2 and <30 kg/m2 and obese = BMI ≥ 30 kg/m2.
cHypertension defined as systolic blood pressure ≥130 mm Hg, diastolic blood pressure ≥80 mm Hg, self-report of high blood pressure diagnosis, or self-report of antihypertensive medication use.
dHyperlipidemia defined as serum total cholesterol ≥200 mg/dL.
eDiabetes defined as defined as a fasting serum glucose ≥120 mg/dL or a nonfasting serum glucose of ≥200 mg/dL, self-report of diabetes diagnosis, or self-report of insulin medication use.
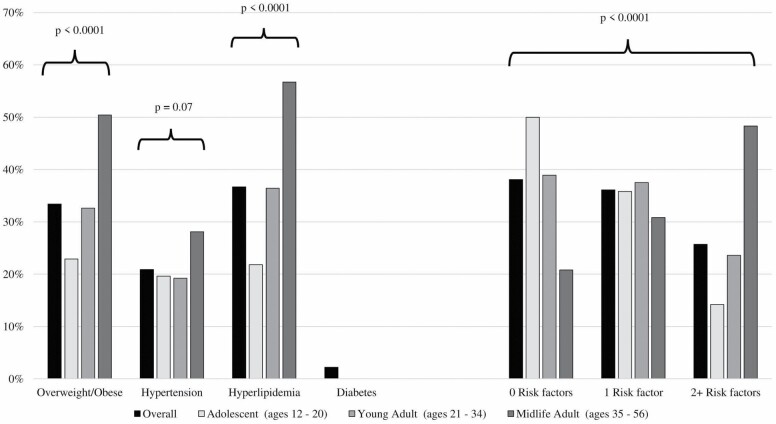
Figure 1.
Prevalence of cardiovascular risk factors stratified by Multiphasic Health Checkup age group.
Overall, the prevalence of CVRFs increased with advancing age (Figure 1). Approximately 50% of adolescents at MHC had no CVRFs versus 39% of young adults and 21% of midlife adults, and these differences were statistically significant (χ2p < .0001). In contrast, only 14% of adolescents had 2 or more CVRFs compared to 24% of young adults and 48% of midlife adults. Similar age trends were seen in individual risk factors. Among adolescents, 23% were overweight or obese versus 33% of young adults and 50% of midlife adults (χ2p < .0001). Twenty-two percent of adolescents had hyperlipidemia compared to 36% of young adults and 57% of midlife adults (χ2p < .0001). For hypertension, prevalence was similar for adolescents (20%) and young adults (19%), but higher in midlife adults (28%) (χ2p = .07). Prevalence of diabetes was low in this sample at 2% overall and was not assessed by MHC age group.
Executive Function
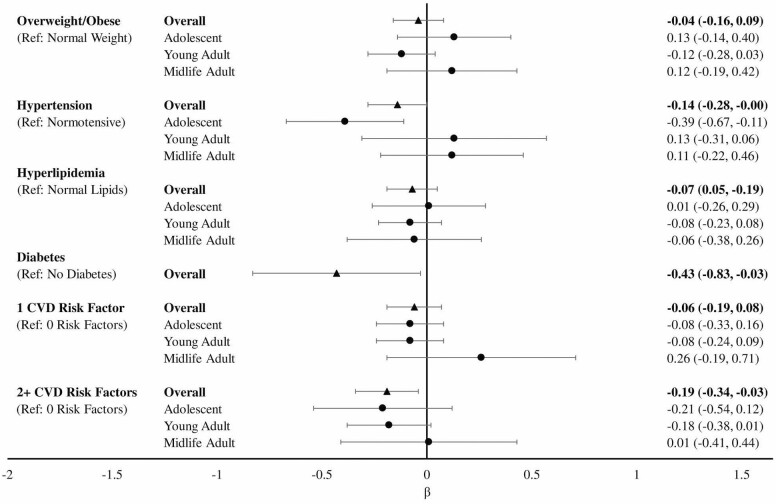
In overall models, hypertension (overall β [95% CI]: −0.14 [−0.28, −0.00003]) and diabetes (overall β [95% CI]: −0.43 [−0.83, −0.03]) were associated with significantly lower executive function z-scores (Figure 2) compared to those without hypertension or diabetes. We found no statistically significant associations between overweight/obesity or hyperlipidemia with late-life executive function. Compared to having no CVRF, having 2 or more risk factors was associated with significantly worse executive function (overall β [95% CI]: −0.19 [−0.34, −0.03]), while having 1 risk factor did not significantly differ (overall β [95% CI]: −0.06 [−0.19, 0.08]). There were no statistically significant CVRFs by age group interactions, suggesting hypertension and diabetes are detrimental to late-life executive function across age groups. Nevertheless, we examined MHC age group-stratified results and found a significant association between adolescent hypertension (β [95% CI]: −0.39 [−0.67, −0.11]) and worse late-life executive function compared to normotensive adolescents.
Figure 2.
Linear regression modelsa of the association between cardiovascular risk factors and executive function overall and stratified by Multiphasic Health Checkup age groupb. aModels adjusted for age at STAR interview, years since Multiphasic Health Checkup, gender, and education. bAdolescent: age 12–20; young adult: age 21–34; midlife adult: age 35–56. STAR = Study of Healthy Aging in African Americans.
Verbal Episodic Memory
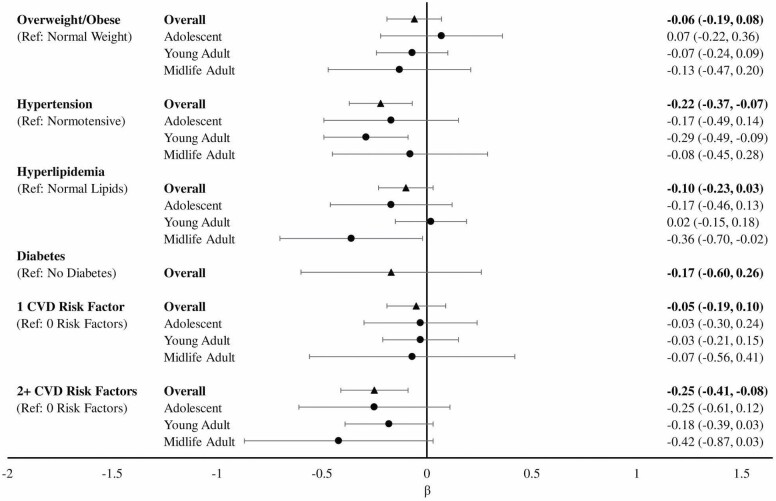
In overall analyses, hypertension was associated with significantly lower late-life verbal episodic memory (overall β [95% CI]: −0.22 [−0.37, −0.07]) compared to those without hypertension (Figure 3). We found no statistically significant associations between overweight/obesity, hyperlipidemia, or diabetes with late-life verbal episodic memory. Compared to those with no CVRFs, having 2 or more risk factors was associated with significantly worse verbal episodic memory (overall β [95% CI]: −0.25 [−0.41, −0.08]) and having 1 risk factor was not significantly associated (overall β [95% CI]: −0.05 [−0.19, 0.10]). There were no statistically significant CVRFs by age group interactions. In MHC age group-stratified analyses, we found significant associations between young adulthood hypertension and worse verbal episodic memory (β [95% CI]: −0.29 [−0.49, −0.09]) compared to young adults without hypertension. We also found that midlife hyperlipidemia was associated with significantly worse verbal episodic memory (β [95% CI]: −0.36 [−0.70, −0.02]) compared to midlife adults without hyperlipidemia.
Figure 3.
Linear regression modelsa of the association between cardiovascular risk factors and verbal episodic memory overall and stratified by Multiphasic Health Checkup age groupb. aModels adjusted for age at STAR interview, years since Multiphasic Health Checkup, gender, and education. bAdolescent: age 12–20; young adult: age 21–34; midlife adult: age 35–56. STAR = Study of Healthy Aging in African Americans.
Semantic Memory
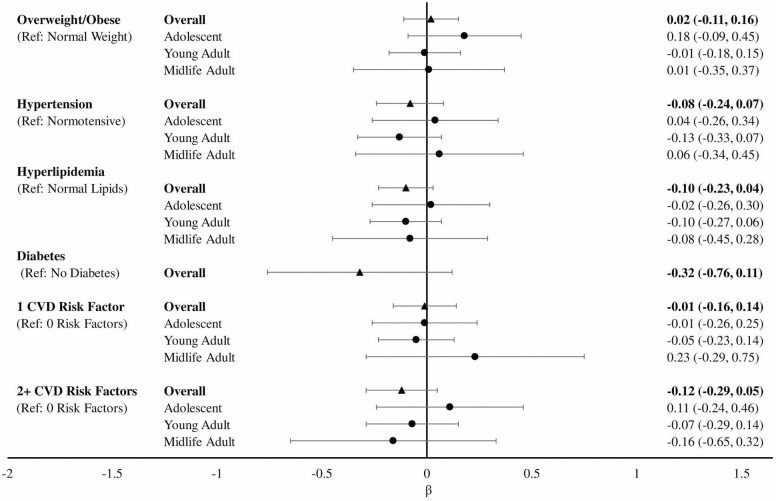
We found no statistically significant associations between individual CVRF or number of CVRF with late-life semantic memory (Figure 4). There was no statistically significant risk factor by age group interactions nor significant associations in MHC age group-stratified analyses.
Figure 4.
Linear regression modelsa of the association between cardiovascular risk factors and semantic memory overall and stratified by Multiphasic Health Checkup age groupb. aModels adjusted for age at STAR interview, years since Multiphasic Health Checkup, gender, and education. bAdolescent: age 12–20; young adult: age 21–34; midlife adult: age 35–56. STAR = Study of Healthy Aging in African Americans.
Additional CVRF Models
We assessed overweight/obesity, hypertension, and hyperlipidemia in the same regression models testing associations with executive function, verbal episodic memory, and semantic memory. When adjusted for in the same regression models, effect estimates for overweight/obesity, hypertension, and hyperlipidemia with each of the 3 cognitive domains were similar to the results described when testing these risk factors individually (Supplementary Table 1). Diabetes was not included due to small numbers.
Discussion
Our study evaluated cognitive aging among community-dwelling Black Americans with exposure data as early as adolescence and cognitive assessments in late life. Consistent with prior literature, prevalence of CVRFs increased with advancing age (43). Almost half of participants who were midlife adults (age 35–56) during their MHC exam had 2 or more CVRFs, while only 24% of young adults (age 21–34) and 14% of adolescents (age 12–20) had multiple CVRFs. Compared to a nationally representative sample of Americans aged 20–49 from the NHANES (1971–1975), prevalence of CVRFs was 0.04%–12.8% lower among STAR participants aged 12–56 (1964–1985) (44). However, age and temporal trends limit comparison of data on life-course CVRFs. There is a dearth of information on CVRFs in Black adults before middle age (10) as well as cognitive aging and dementia among Black Americans (45), which is why studies like STAR are so important.
Midlife overweight/obesity, hypertension, and hypercholesteremia, as well as mid- and late-life diabetes have been identified as risk factors for cognitive decline and dementia (15,22,23,46–49). A limitation of these studies is that they primarily focused on individuals aged 40 and older and did not assess risk factors developed earlier in the life course (22,23,50–52). Given the long preclinical and prodromal periods of cognitive impairment and dementia, public health interventions on CVRFs may be needed decades prior to the onset of symptoms (53–56). Timing of risk factors in relation to timing of cognitive impairment and dementia is severely understudied, particularly in diverse populations where CRVF burden is disproportionately higher. Neuropathological studies have found that among Black Americans, there is a disproportionate burden of cerebrovascular disease compared to other racial/ethnic groups, which makes understanding the contributions of CVRFs to late-life cognition particularly important for older Black adults (57,58).
In age-stratified models, we found that midlife (age 35–56) hyperlipidemia was associated with worse verbal episodic memory, a finding consistent with the literature (22). Hypertension present in adolescence (age 12–20) was associated with significantly worse executive function in late life, and hypertension in young adulthood (age 21–34) was associated with significantly worse verbal episodic memory. Previous research has identified young adulthood hypertension as a risk factor for dementia (59). Deficits in episodic memory and executive function are hallmark features of dementia, while semantic memory is often only partially impacted, particularly in Alzheimer’s disease (60,61). While midlife appears to be a sensitive period for CVRFs (22,23,62,63), particularly hypertension (64–66), our analysis suggests that this sensitive period may start as early as adolescence (age 12–20) or young adulthood (age 21–34). Screening for CVRFs and treatment through medication and lifestyle interventions throughout the life course is not only important for maintaining cardiovascular health, but also the maintenance of late-life brain health in Black Americans (67). This group is more likely to experience chronic stress and weathering that may contribute to the development of CVRFs at earlier ages (68,69) and, may, in part, be driving racial/ethnic disparities in late-life cognitive outcomes (70).
Our study has several strengths including a large number of Black participants with diverse ethnic and educational backgrounds (18% high school or less, 47% some college, vocational, or trade school, and 35% college graduate or more education). The wide range of ages at which individuals first participated in an MHC exam enables us to examine possible sensitive periods between CVRFs, as early as adolescence, with late-life cognition. MHC exams are a source of prospectively collected clinical and self-reported measures of CVRFs. Another strength is the assessment of cognitive function using the SENAS, a comprehensive and sensitive assessment tool that has been validated across diverse racial/ethnic groups. Over 40 years elapsed between assessment of CVRFs at MHC and measurement of cognition in STAR ensuring that risk factors were captured well before the onset of any age-related changes or disease.
Despite these strengths, there were several limitations including limited power to assess statistically significant differences in the association between CVRFs and cognition across MHC age groups. Due to the distribution of participant ages at MHC, the young adults were overrepresented while adolescents and midlife adults were underrepresented, limiting power to test for MHC age group interactions. However, we did see evidence of heterogeneity across age groups that warrants further investigation. We were also limited to measurement of CVRFs at one time point and thus compared individuals at different ages at CVRF measurement versus following the same person over time. Similarly, cognition was only available at one time point, which did not allow us to assess cognitive decline or impairment. Finally, we were unable to adjust for social determinants of health beyond participant education and parental education. We used education as a proxy for socioeconomic status; however, there are many other determinants that may influence the effect of CVRFs on cognition. As we continue to collect data, future research in this cohort will assess CVRFs at multiple time points in the life course, characterize life-course patterns of risk factors, and evaluate cognitive decline.
Black Americans are at high risk of developing common CVRFs including overweight/obesity, hyperlipidemia, hypertension, and diabetes (1,2,5,9,10). Prevalence of these risk factors over the life course increases with advancing age and can have a negative impact on late-life cognitive function even when developed in adolescence or young adulthood. Public health initiatives to reduce the burden of CVRFs among Black Americans need to include individuals from adolescence through midlife. These modifiable risk factors provide an opportunity for risk reduction through targeted treatment and management in a population at excess risk due to experiences of racism and its impact on the body through weathering. Treatment and management of CVRFs will not only help in reducing risk of CVD but may also prevent detrimental effects of CVRFs on late-life brain health.
Funding
K.M.G. was supported by the National Institutes of Health (NIH)/National Institute on Aging (NIA) Neuroscience of Cognitive Aging Training Grant (T32AG050061). This work was funded by the NIH/NIA under grant numbers RF1AG05078202 (PI: R.A.W.) and R00AG053410 (PI: E.R.M.).
Conflict of Interest
None declared.
Supplementary Material
References
- 1.Pool LR, Ning H, Lloyd-Jones DM, Allen NB. Trends in racial/ethnic disparities in cardiovascular health among US adults from 1999–2012. J Am Heart Assoc. 2017;6(9):e006027. doi: 10.1161/JAHA.117.006027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Health, United States Spotlight: Racial and Ethnic Disparities in Heart Disease. 2019. https://www.cdc.gov/nchs/hus/spotlight/HeartDiseaseSpotlight_2019_0404.pdf. Accessed February 9, 2020.
- 3.Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol. 2016;35(4):407–411. doi: 10.1037/hea0000242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96(5):826–833. doi: 10.2105/AJPH.2004.060749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Effoe VS, Carnethon MR, Echouffo-Tcheugui JB, et al. The American Heart Association ideal cardiovascular health and incident type 2 diabetes mellitus among blacks: the Jackson Heart Study. J Am Heart Assoc. 2017;6(6):e005008. doi: 10.1161/JAHA.116.005008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xanthakis V, Sung JH, Samdarshi TE, et al. Relations between subclinical disease markers and type 2 diabetes, metabolic syndrome, and incident cardiovascular disease: the Jackson Heart Study. Diabetes Care. 2015;38(6):1082–1088. doi: 10.2337/dc14-2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas AJ, Eberly LE, Davey Smith G, Neaton JD, Stamler J. Race/ethnicity, income, major risk factors, and cardiovascular disease mortality. Am J Public Health. 2005;95(8):1417–1423. doi: 10.2105/AJPH.2004.048165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George KM, Folsom AR, Steffen LM, Wagenknecht LE, Mosley TH. Differences in cardiovascular mortality risk among African Americans in the Minnesota Heart Survey: 1985–2015 vs The Atherosclerosis Risk in Communities Study Cohort: 1987–2015. Ethn Dis. 2019;29(1):47–52. doi: 10.18865/ed.29.1.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong ND, Zhao Y, Patel R, et al. Cardiovascular risk factor targets and cardiovascular disease event risk in diabetes: a pooling project of the atherosclerosis risk in communities study, multi-ethnic study of atherosclerosis, and Jackson Heart Study. Diabetes Care. 2016;39(5):668–676. doi: 10.2337/dc15-2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ommerborn MJ, Blackshear CT, Hickson DA, et al. Ideal cardiovascular health and incident cardiovascular events: the Jackson Heart Study. Am J Prev Med. 2016;51(4): 502–506. doi: 10.1016/j.amepre.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SPRINT Mind Investigators for the SPRINT Research Group, Williamson JD, Pajewski NM, et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. J Am Med Assoc. 2019;321(6):553–561. doi: 10.1001/jama.2018.21442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol. 2018;14(10):591–604. doi: 10.1038/s41574-018-0048-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev. 2011;12(5):e426–e437. doi: 10.1111/j.1467-789X.2010.00825.x [DOI] [PubMed] [Google Scholar]
- 14.Anstey KJ, Lipnicki DM, Low LF. Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry. 2008;16(5):343–354. doi: 10.1097/JGP.0b013e31816b72d4 [DOI] [PubMed] [Google Scholar]
- 15.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62(10):1556–1560. doi: 10.1001/archneur.62.10.1556 [DOI] [PubMed] [Google Scholar]
- 16.Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord. 2009;28(1):75–80. doi: 10.1159/000231980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kivipelto M, Helkala EL, Laakso MP, et al. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med. 2002;137(3):149–155. doi: 10.7326/0003-4819-137-3-200208060-00006 [DOI] [PubMed] [Google Scholar]
- 18.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12(3):216–224. doi: 10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weuve J, Barnes LL, Mendes de Leon CF, et al. Cognitive aging in black and white Americans: cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiology. 2018;29(1):151–159. doi: 10.1097/EDE.0000000000000747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zahodne LB, Manly JJ, Azar M, Brickman AM, Glymour MM. Racial disparities in cognitive performance in mid- and late adulthood: analyses of two cohort studies. J Am Geriatr Soc. 2016;64(5):959–964. doi: 10.1111/jgs.14113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corriveau RA, Koroshetz WJ, Gladman JT, et al. Alzheimer’s disease-related dementias summit 2016: national research priorities. Neurology. 2017;89(23):2381–2391. doi: 10.1212/WNL.0000000000004717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64(2):277–281. doi: 10.1212/01.WNL.0000149519.47454.F2 [DOI] [PubMed] [Google Scholar]
- 23.Knopman DS, Gottesman RF, Sharrett AR, et al. Midlife vascular risk factors and midlife cognitive status in relation to prevalence of mild cognitive impairment and dementia in later life: The Atherosclerosis Risk in Communities Study. Alzheimers Dement. 2018;14(11):1406–1415. doi: 10.1016/j.jalz.2018.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Exalto LG, Quesenberry CP, Barnes D, Kivipelto M, Biessels GJ, Whitmer RA. Midlife risk score for the prediction of dementia four decades later. Alzheimers Dement. 2014;10(5):562–570. doi: 10.1016/j.jalz.2013.05.1772 [DOI] [PubMed] [Google Scholar]
- 25.Mungas D, Reed BR, Marshall SC, Gonzalez HM. Development of psychometrically matched English and Spanish language neuropsychological tests for older persons. Neuropsychology. 2000;14(2):209–223. doi: 10.1037//0894-4105.14.2.209 [DOI] [PubMed] [Google Scholar]
- 26.Mungas D, Reed BR, Crane PK, Haan MN, Gonzalez H. Spanish and English Neuropsychological Assessment Scales (SENAS): further development and psychometric characteristics. Psychol Assess. 2004;16(4):347–359. doi: 10.1037/1040-3590.16.4.347 [DOI] [PubMed] [Google Scholar]
- 27.Mungas D, Beckett L, Harvey D, et al. Heterogeneity of cognitive trajectories in diverse older persons. Psychol Aging. 2010;25(3):606–619. doi: 10.1037/a0019502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mungas D, Widaman KF, Reed BR, Tomaszewski Farias S. Measurement invariance of neuropsychological tests in diverse older persons. Neuropsychology. 2011;25(2):260–269. doi: 10.1037/a0021090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mungas D, Reed BR, Haan MN, Gonzalez H. Spanish and English Neuropsychological Assessment Scales: relationship to demographics, language, cognition, and independent function. Neuropsychology. 2005;19(4):466–475. doi: 10.1037/0894-4105.19.4.466 [DOI] [PubMed] [Google Scholar]
- 30.Mungas D, Reed BR, Tomaszewski Farias S, DeCarli C. Criterion-referenced validity of a neuropsychological test battery: equivalent performance in elderly Hispanics and non-Hispanic Whites. J Int Neuropsychol Soc. 2005;11(5):620–630. doi: 10.1017/S1355617705050745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crane PK, Narasimhalu K, Gibbons LE, et al. Composite scores for executive function items: demographic heterogeneity and relationships with quantitative magnetic resonance imaging. J Int Neuropsychol Soc. 2008;14(5):746–759. doi: 10.1017/S1355617708081162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Early DR, Widaman KF, Harvey D, et al. Demographic predictors of cognitive change in ethnically diverse older persons. Psychol Aging. 2013;28(3):633–645. doi: 10.1037/a0031645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brewster PW, Melrose RJ, Marquine MJ, et al. Life experience and demographic influences on cognitive function in older adults. Neuropsychology. 2014;28(6):846–858. doi: 10.1037/neu0000098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melrose RJ, Brewster P, Marquine MJ, et al. Early life development in a multiethnic sample and the relation to late life cognition. J Gerontol B Psychol Sci Soc Sci. 2015;70(4):519–531. doi: 10.1093/geronb/gbt126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mungas D, Reed BR, Farias ST, Decarli C. Age and education effects on relationships of cognitive test scores with brain structure in demographically diverse older persons. Psychol Aging. 2009;24(1):116–128. doi: 10.1037/a0013421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fletcher E, Gavett B, Harvey D, et al. Brain volume change and cognitive trajectories in aging. Neuropsychology. 2018;32(4):436–449. doi: 10.1037/neu0000447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gavett BE, Fletcher E, Harvey D, et al. Ethnoracial differences in brain structure change and cognitive change. Neuropsychology. 2018;32(5):529–540. doi: 10.1037/neu0000452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reed BR, Mungas D, Farias ST, et al. Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain. 2010;133(Pt 8):2196–2209. doi: 10.1093/brain/awq154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mungas D, Early DR, Glymour MM, Zeki Al Hazzouri A, Haan MN. Education, bilingualism, and cognitive trajectories: Sacramento Area Latino Aging Study (SALSA). Neuropsychology. 2018;32(1):77–88. doi: 10.1037/neu0000356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mungas D, Gavett B, Fletcher E, Farias ST, DeCarli C, Reed B. Education amplifies brain atrophy effect on cognitive decline: implications for cognitive reserve. Neurobiol Aging. 2018;68:142–150. doi: 10.1016/j.neurobiolaging.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bettcher BM, Gross AL, Gavett BE, et al. Dynamic change of cognitive reserve: associations with changes in brain, cognition, and diagnosis. Neurobiol Aging. 2019;83:95–104. doi: 10.1016/j.neurobiolaging.2019.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mungas D, Fletcher E, Gavett BE, et al. Comparison of education and episodic memory as modifiers of brain atrophy effects on cognitive decline: implications for measuring cognitive reserve. J Int Neuropsychol Soc. 2021; 27(5):401–411. doi: 10.1017/S1355617720001095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costantino S, Paneni F, Cosentino F. Ageing, metabolism and cardiovascular disease. J Physiol. 2016;594(8):2061–2073. doi: 10.1113/JP270538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casagrande SS, Menke A, Cowie CC. Cardiovascular risk factors of adults age 20–49 years in the United States, 1971–2012: a series of cross-sectional studies. PLoS One. 2016;11(8):e0161770. doi: 10.1371/journal.pone.0161770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA. The Minority Aging Research Study: ongoing efforts to obtain brain donation in African Americans without dementia. Curr Alzheimer Res. 2012;9(6):734–745. doi: 10.2174/156720512801322627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rawlings AM, Sharrett AR, Albert MS, et al. The association of late-life diabetes status and hyperglycemia with incident mild cognitive impairment and dementia: the ARIC study. Diabetes Care. 2019;42(7):1248–1254. doi: 10.2337/dc19-0120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayeda ER, Karter AJ, Huang ES, Moffet HH, Haan MN, Whitmer RA. Racial/ethnic differences in dementia risk among older type 2 diabetic patients: the Diabetes and Aging Study. Diabetes Care. 2014;37(4):1009–1015. doi: 10.2337/dc13-0215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitmer RA, Gunderson EP, QuesenberryCP, Jr., Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res. 2007;4(2):103–109. doi: 10.2174/156720507780362047 [DOI] [PubMed] [Google Scholar]
- 49.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP Jr., Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. Br Med J. 2005;330(7504):1360. doi: 10.1136/bmj.38446.466238.E0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alonso A, JacobsDR, Jr., Menotti A, et al. Cardiovascular risk factors and dementia mortality: 40 years of follow-up in the Seven Countries Study. J Neurol Sci. 2009;280(1–2):79-83. doi: 10.1016/j.jns.2009.02.004 [DOI] [PubMed] [Google Scholar]
- 51.Tolppanen AM, Solomon A, Soininen H, Kivipelto M. Midlife vascular risk factors and Alzheimer’s disease: evidence from epidemiological studies. J Alzheimers Dis. 2012;32(3):531–540. doi: 10.3233/JAD-2012-120802 [DOI] [PubMed] [Google Scholar]
- 52.Yaffe K, Bahorik AL, Hoang TD, et al. Cardiovascular risk factors and accelerated cognitive decline in midlife: The CARDIA Study. Neurology. 2020;95(7):e839–e846. doi: 10.1212/WNL.0000000000010078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Twamley EW, Ropacki SA, Bondi MW. Neuropsychological and neuroimaging changes in preclinical Alzheimer’s disease. J Int Neuropsychol Soc. 2006;12(5):707–735. doi: 10.1017/S1355617706060863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vermunt L, Sikkes SAM, van den Hout A, et al. ; Alzheimer Disease Neuroimaging Initiative; AIBL Research Group; ICTUS/DSA Study Groups . Duration of preclinical, prodromal, and dementia stages of Alzheimer’s disease in relation to age, sex, and APOE genotype. Alzheimers Dement. 2019;15(7):888–898. doi: 10.1016/j.jalz.2019.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dubois B, Hampel H, Feldman HH, et al. ; Proceedings of the Meeting of the International Working Group (IWG) and the American Alzheimer’s Association on “The Preclinical State of AD”; July 23, 2015; Washington DC, USA . Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12(3):292–323. doi: 10.1016/j.jalz.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Epelbaum S, Genthon R, Cavedo E, et al. Preclinical Alzheimer’s disease: a systematic review of the cohorts underlying the concept. Alzheimers Dement. 2017;13(4):454–467. doi: 10.1016/j.jalz.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 57.Filshtein TJ, Dugger BN, Jin LW, et al. Neuropathological diagnoses of demented Hispanic, black, and non-Hispanic white decedents seen at an Alzheimer’s disease center. J Alzheimers Dis. 2019;68(1):145–158. doi: 10.3233/JAD-180992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barnes LL, Leurgans S, Aggarwal NT, et al. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology. 2015;85(6):528–534. doi: 10.1212/WNL.0000000000001834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gilsanz P, Mayeda ER, Glymour MM, et al. Female sex, early-onset hypertension, and risk of dementia. Neurology. 2017;89(18):1886–1893. doi: 10.1212/WNL.0000000000004602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolk DA, Dickerson BC; Alzheimer’s Disease Neuroimaging Initiative . Fractionating verbal episodic memory in Alzheimer’s disease. Neuroimage. 2011;54(2):1530–1539. doi: 10.1016/j.neuroimage.2010.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rogers SL, Friedman RB. The underlying mechanisms of semantic memory loss in Alzheimer’s disease and semantic dementia. Neuropsychologia. 2008;46(1):12–21. doi: 10.1016/j.neuropsychologia.2007.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Conner SC, Pase MP, Carneiro H, et al. Mid-life and late-life vascular risk factor burden and neuropathology in old age. Ann Clin Transl Neurol. 2019;6(12):2403–2412. doi: 10.1002/acn3.50936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pedditzi E, Peters R, Beckett N. The risk of overweight/obesity in mid-life and late life for the development of dementia: a systematic review and meta-analysis of longitudinal studies. Age Ageing. 2016;45(1):14–21. doi: 10.1093/ageing/afv151 [DOI] [PubMed] [Google Scholar]
- 64.Pase MP, Davis-Plourde K, Himali JJ, et al. Vascular risk at younger ages most strongly associates with current and future brain volume. Neurology. 2018;91(16):e1479–e1486. doi: 10.1212/WNL.0000000000006360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. J Am Med Assoc. 1995;274(23):1846–1851. [PubMed] [Google Scholar]
- 66.Walker KA, Power MC, Gottesman RF. Defining the relationship between hypertension, cognitive decline, and dementia: a review. Curr Hypertens Rep. 2017;19(3):24. doi: 10.1007/s11906-017-0724-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lazar RM, Howard VJ, Kernan WN, et al. A primary care agenda for brain health: a scientific statement from the American Heart Association. Stroke. 2021:STR0000000000000367. doi: 10.1161/STR.0000000000000367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sumner AE. Ethnic differences in triglyceride levels and high-density lipoprotein lead to underdiagnosis of the metabolic syndrome in black children and adults. J Pediatr. 2009;155(3):S7.e7–S7.11. doi: 10.1016/j.jpeds.2009.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walker SE, Gurka MJ, Oliver MN, Johns DW, DeBoer MD. Racial/ethnic discrepancies in the metabolic syndrome begin in childhood and persist after adjustment for environmental factors. Nutr Metab Cardiovasc Dis. 2012;22(2):141–148. doi: 10.1016/j.numecd.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Babulal GM, Quiroz YT, Albensi BC, et al. ; International Society to Advance Alzheimer’s Research and Treatment, Alzheimer’s Association . Perspectives on ethnic and racial disparities in Alzheimer’s disease and related dementias: update and areas of immediate need. Alzheimers Dement. 2019;15(2):292–312. doi: 10.1016/j.jalz.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.