Fig. 1.
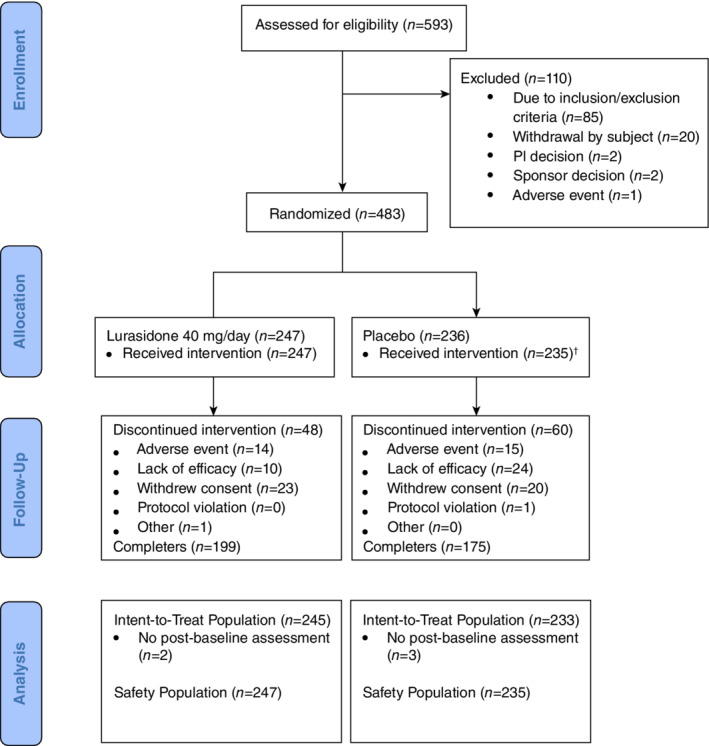
Patient disposition. †One subject did not receive study drug due to an important protocol deviation in the placebo group and was excluded from the intent‐to‐treat (ITT) and safety population. PI, principal investigator.

Patient disposition. †One subject did not receive study drug due to an important protocol deviation in the placebo group and was excluded from the intent‐to‐treat (ITT) and safety population. PI, principal investigator.