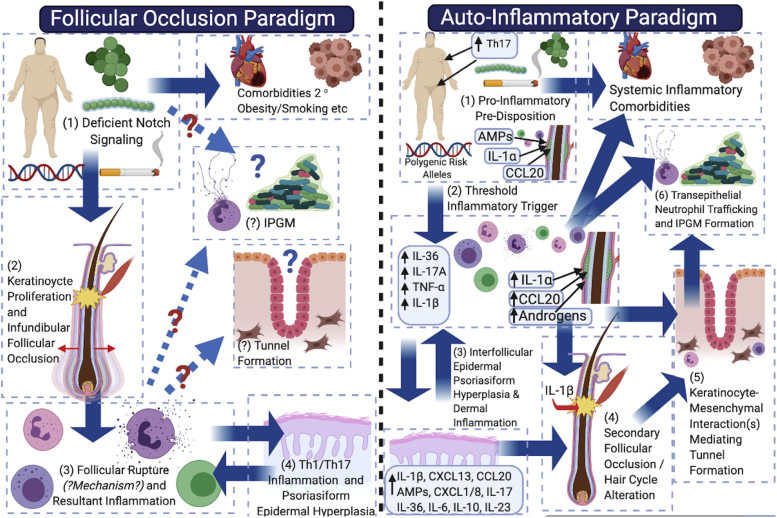
Fig 1.
Schematic representation and comparison of the follicular occlusion paradigm (left panel) and the autoinflammatory paradigm (right panel) in the pathogenesis of hidradenitis suppurativa. In the follicular occlusion paradigm, deficient Notch signaling (1) directly results in infundibular keratinocyte proliferation and follicular occlusion (2), leading to follicular dilatation, rupture, and resultant inflammation. One deficiency of this paradigm is the lack of hypothesized mechanisms by which rupture occurs and why deep follicular rupture occurs preferentially to expulsion of the comedo. The resultant T-helper cell 1/17 inflammatory axis (3) (4) then results in the observed inflammatory profile of disease; however, no clear mechanism is hypothesized for how tunnels form and how the infiltrative proliferative gelatinous mass results. The autoinflammatory paradigm (right panel) places inflammation as the primary driver of disease, with subclinical inflammation (1) developing as a result of disparate contributing factors on a background of topographic predisposition. Dermal inflammatory infiltrates (2) then drive secondary follicular occlusion (3 and 4), with resultant tunnel formation a consequence of keratinocyte-mesenchymal interactions (5) that mimic outer-root sheath keratinocyte downgrowth in follicular development in early anagen. Chemokine gradients in epithelialized tunnels then drive neutrophil trafficking to the lumen and formation of the infiltrative proliferative gelatinous mass (6).