Figure 1.
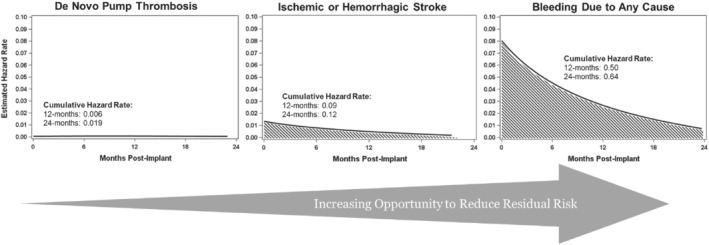
Residual risk of haemocompatibility‐related adverse effects with the HeartMate 3 (HM3) left ventricular assist device (LVAD). The figure depicts estimated hazard rates for device thrombosis, stroke and bleeding events in the HM3 arm of the MOMENTUM 3 pivotal trial.6 Hazard rates to first event are calculated using kernel‐smoothed hazard function. With the HM3, pump thrombosis has become a rare event. For stroke and bleeding, >75% of the cumulative hazard occurs within the first year of LVAD support, representing the greatest opportunity to reduce residual risk in HM3 patients.
