FIGURE 2.
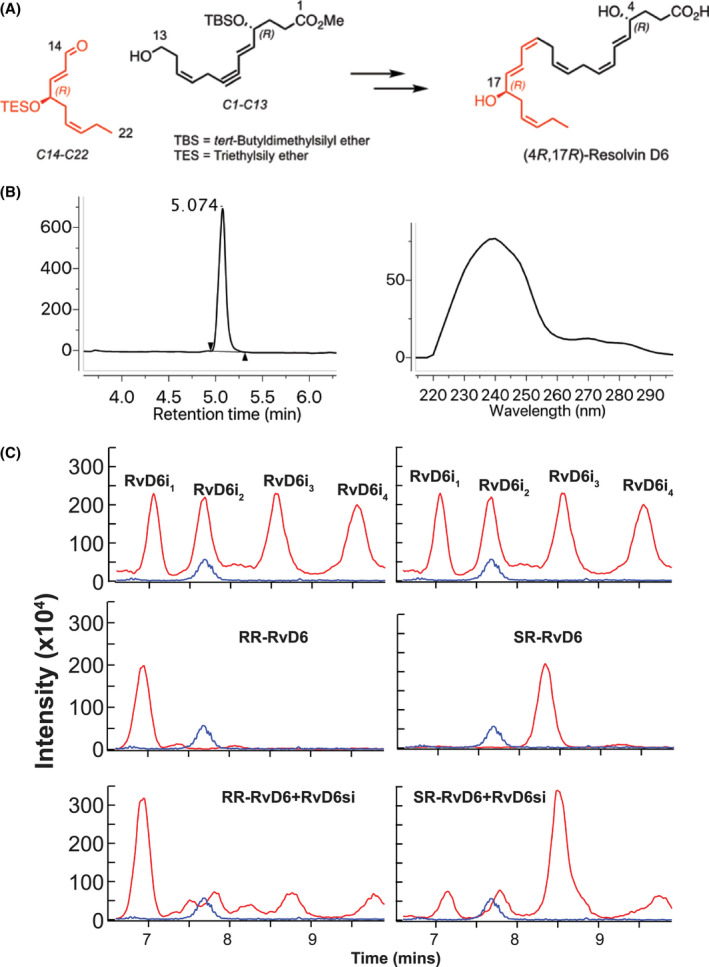
The structure of RvD6si was confirmed by total synthesis. A, The synthetic scheme of RR‐RvD6 involved key synthetic intermediates C1‐C13 (black) and C14‐C22 (red) were constructed from chirally pure building blocks that were synthesized in conjunction with stereospecific transformations to furnish the target with absolute stereo‐ and regiochemical control. B. The purity of RR‐RvD6 was confirmed by LC‐MS chromatogram (left) while its characteristic conjugated 1,3‐butadiene moieties are shown in the molecule at carbon positions C5‐C7 and C13‐C15, confirmed by UV spectrum (right). B, LC‐MS/MS analysis of mouse tears‐derived RvD6si using a chiral column shows the separation of four peaks. Chemical synthetized RR‐RvD6 had the same retention time as the first peak of RvD6si, while the SR‐RvD6 partially matched the third peak of RvD6 isomer. Only RR‐RvD6 has an earlier retention time than the internal standard LTB4‐d4 (blue), as shown in Figure 1A
