Fig. 1. Multi-antigen prime-and-kill circuits in T cells provide a general strategy to overcome antigen heterogeneity while still maintaining high tumor specificity.
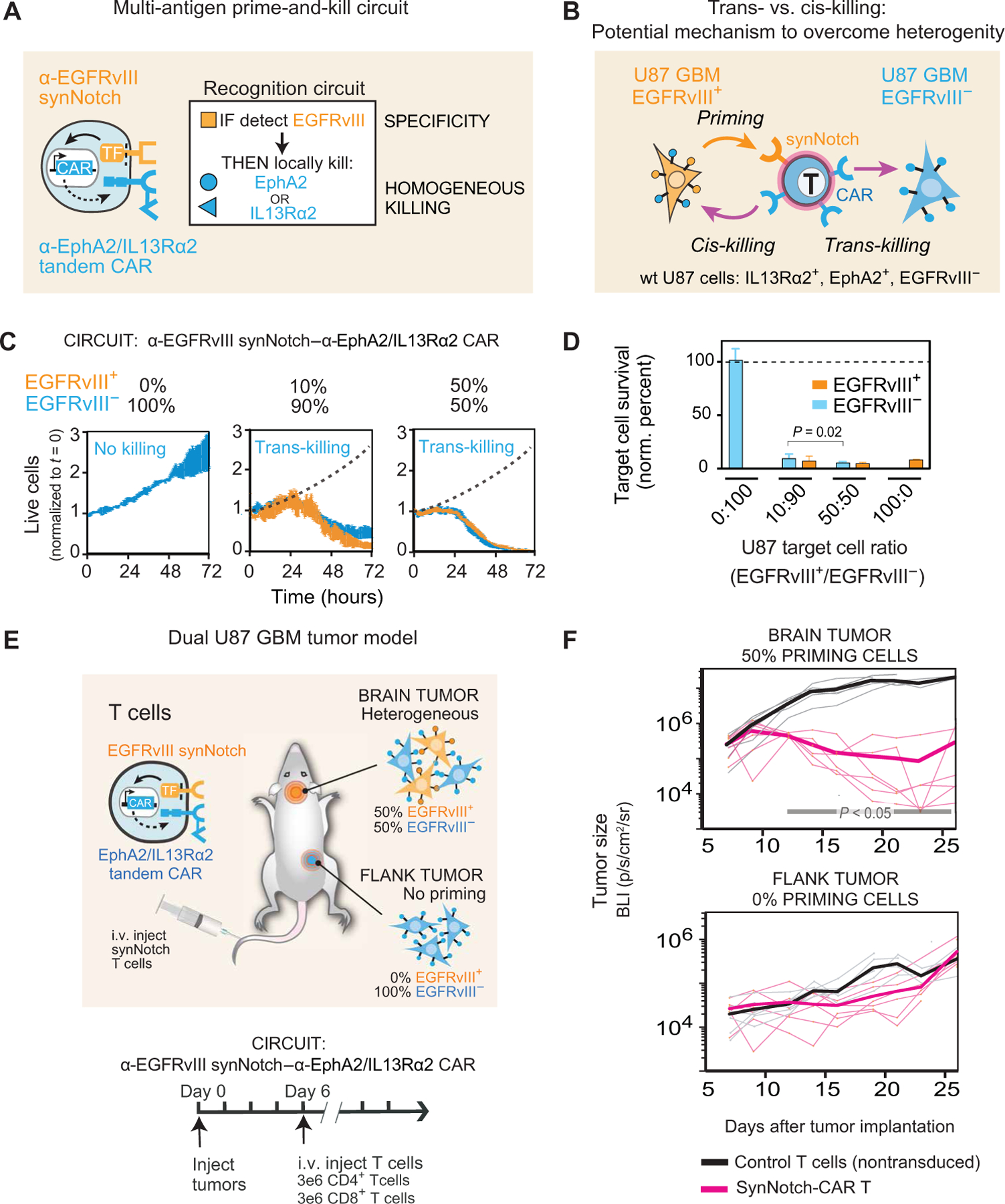
(A) Design of synNotch-CAR circuit primed by EGFRvIII neoantigen: α-EGFRvIII synNotch receptor induces expression of tandem α-EphA2/IL13Rα2 CAR (TF, transcription factor). These cells should be activated to kill EphA2+ or IL13Rα2+ target cells only if exposed to EGFRvIII+ cells. (B) Such T cells could overcome priming antigen heterogeneity if they can execute trans-killing, where priming and killing antigens are expressed on different but neighboring cells. (C) Real-time killing assays using heterogeneous mixtures of EGFRvIII+ and EGFRvIII− target cells show efficient trans-killing. Primary CD8+ human T cells transduced with α-EGFRvIII synNotch–α-EphA2/IL13Rα2 CAR circuit were cultured with indicated ratios of EGFRvIII+ versus EGFRVIII− U87 cells at an E:T ratio of 5:1 and imaged over 3 days using IncuCyte. The EGFRvIII+ cell population (priming cells) is shown in yellow, and the EGFRvIII− cell population (target cells) is shown in blue. The presence of as low as 10% priming cells yielded strong killing of EGFRvIII− target cells, although killing was slightly slower compared to that observed with 50% priming cells (P = 0.0149 and P = 0.0218, t test at 48 and 72 hours, respectively). The dotted black line shows the growth of 100% target cells as a reference (n = 3, error bars denote SEM). See movie S1. (D) Relative survival of each cell type in experiments from (C) (at 72 hours). Killing of target cells is efficiently primed by as low as 10% priming cells (EGFRvIII+). No killing is observed in the absence of priming cells (n = 3, error bars denote SD). A t test was used for statistical comparison. (E) NCG mice were simultaneously implanted with two GBM tumors: a heterogeneous tumor comprising EGFRvIII+ and EGFRvIII− U87 cells (1:1 ratio) in the brain, and a homogeneous EGFRvIII− U87 tumor implanted subcutaneously in the flank. Mice were treated 6 days after tumor implantation with intravenous (i.v.) infusion of 3 million CD4+ and CD8+ synNotch-CAR T cells (n = 6) or control nontransduced T cells (n = 6). (F) Tumor size was measured by luciferase bioluminescence imaging (BLI) over time as the number of photons per second per square centimeter per steradian (p/s/cm2/sr). Tumor size curves for individual mice treated with synNotch-CAR T cells are shown in light pink; curves for mice treated with nontransduced T cells are shown in gray. Thicker lines correspond to geometric means. P < 0.05 by Mann-Whitney test on day 12 and onward, whereas the flank tumor grew at the same rate as in the mice treated with nontransduced T cells.
