Fig. 2. SynNotch-CAR T cells show improved efficacy and durability compared to individual parental constitutive CARs in clearing heterogeneous GBM6 PDX tumors.
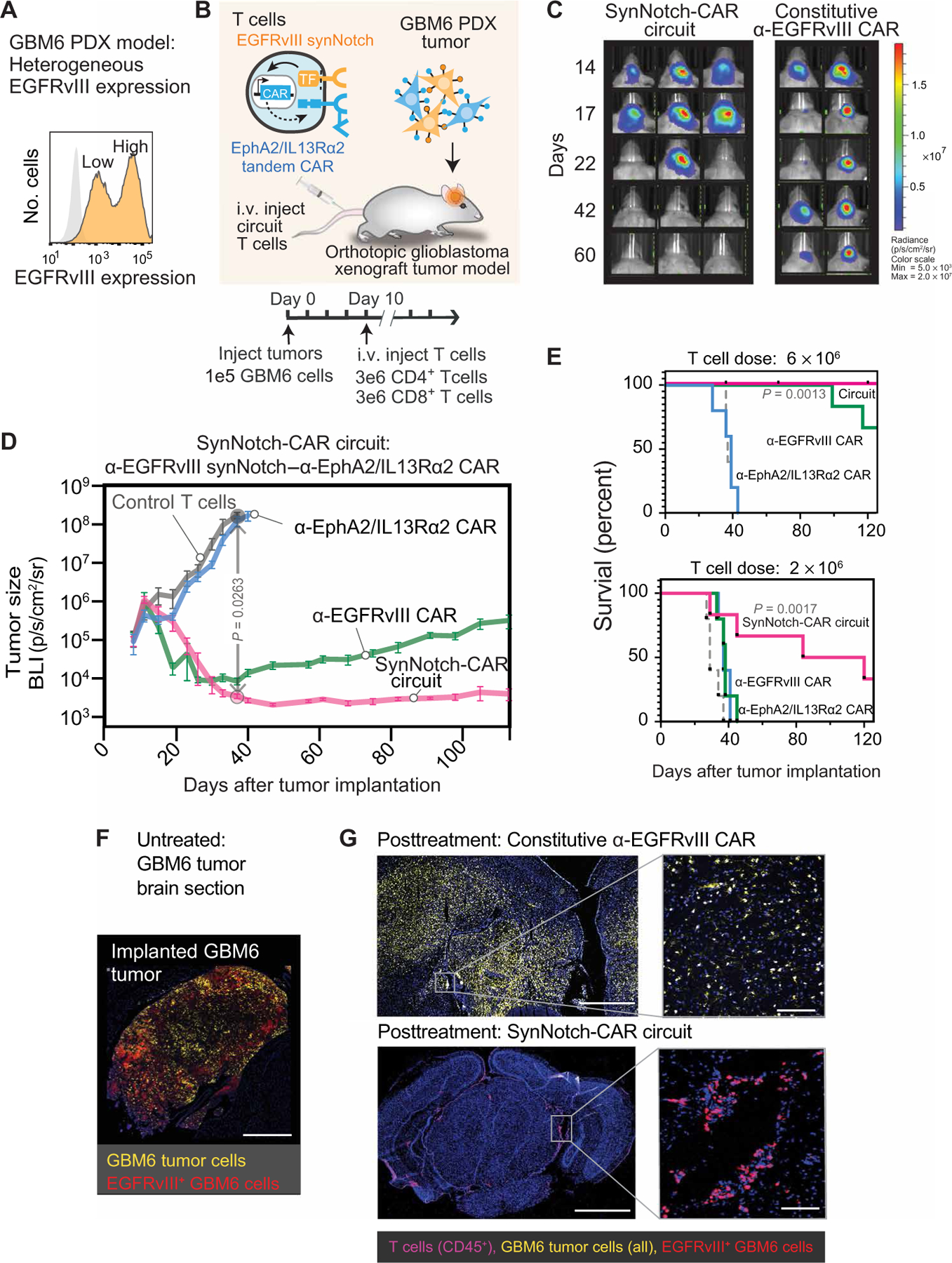
(A) Flow cytometry analysis of a patient-derived xenograft (PDX) GBM6 tumor model shows intrinsic heterogeneity of EGFRvIII expression. (B) Timeline for in vivo tumor experiments with GBM6 tumors. GBM6 tumors expressing mCherry and luciferase were orthotopically implanted in brains of NCG mice. Ten days after tumor implantation, mice were infused intravenously (i.v.) with 3 million each of CD4+ and CD8+ T cells expressing no construct (control) (n = 5), α-EGFRvIII synNotch–α-EphA2/IL13Rα2 CAR circuit (n = 6), constitutively expressed α-EGFRvIII CAR (n = 5), or constitutively expressed α-EphA2/IL13Rα2 tandem CAR (n = 5). (C) Longitudinal bioluminescence imaging of GBM6 tumor–bearing mice treated with α-EGFRvIII synNotch–α-EphA2/IL13Rα2 CAR T cells and conventional α-EGFRvIII CAR T cells. Each column represents one mouse over time. (D) Time course of tumor size measured by bioluminescence. P = 0.0263, two-way ANOVA followed by a Dunnett’s test nontransduced versus synNotch-CAR T cells at day 37. Error bars represent means ± SEM of five to six individual mice from one experiment. (E) Kaplan-Meier survival curves for high-dose (6 × 106 cells) and low-dose (2 × 106 cells) treatments. Statistical significance was calculated using log-rank Mantel-Cox test. (F) Fluorescence microscopy of representative section of untreated GBM6 xenograft tumor, isolated 15 days after tumor implantation, shows heterogeneous expression of EGFRvIII. Scale bar, 500 µm. (G) Top: Representative fluorescence microscopy of a brain and tumor section isolated 107 days after treatment with conventional α-EGFRvIII CAR T cells shows the presence of tumor but loss of EGFRvIII expression. Bottom: Representative fluorescence microscopy of a brain section isolated 110 days after treatment with EGFRvIII synNotch–α-EphA2/IL13Rα2 CAR T cells reveals clearance of a GBM6 xenograft tumor and sustained presence of T cells. Scale bars, 1 mm (left) and 50 µm (right).
