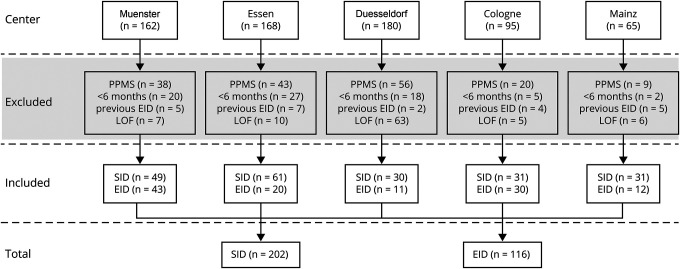
Figure 1. Flowchart of Case Ascertainment.
This flowchart depicts how the 318 ocrelizumab-treated patients with relapsing-remitting multiple sclerosis (RRMS) were identified. The source population was all patients with multiple sclerosis (MS) treated with ocrelizumab in 5 German centers during the period between January 2020 and September 2020. We excluded patients with MS who were treated with ocrelizumab due to a primary progressive disease course (PPMS), if only the 2 induction cycles with 300 mg were administered (treatment duration with ocrelizumab <6 months (Mo) during the observational period). SID and EID do not refer to the first 2 half doses with 300 mg, respectively, if patients experienced an extended interval dosing (EID) before Infusion C (the infusion administered between January 2020 and September 2020 that led to the division of the 2 groups–standard interval dosing [SID] and EID), or if the patient for any other reason did not satisfy the inclusion criteria (e.g., loss of follow-up [LOF]).