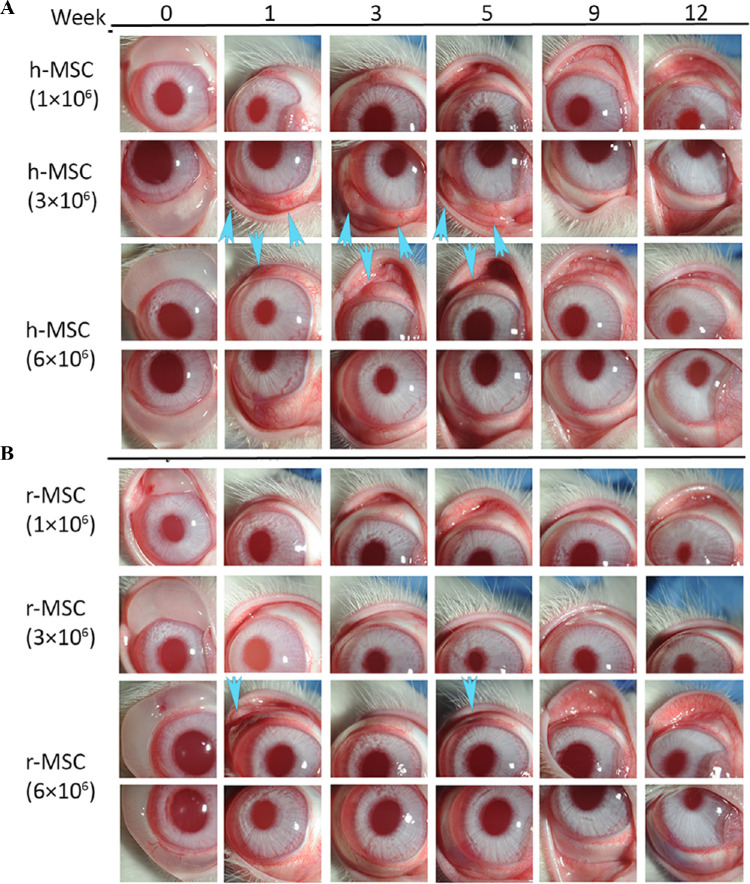
Figure 3.
Subconjunctival injection of human or rabbit bone marrow MSCs into uninjured rabbit eyes. Human (A) or rabbit (B) bone marrow–derived MSCs were subconjunctivally injected in rabbits at different doses and were followed up to 12 weeks to evaluate the safety. Some rabbit eyes injected with higher doses of human MSCs showed mild redness and swelling in the conjunctival injection area until week 6 (arrows), which resolved by week 12. This pattern was also observed on the first week when rabbit corneas were injected with higher concentration (6 × 106) of rabbit MSCs. In weekly follow-ups, there was no abnormal finding including corneal epithelial defects, corneal neovascularization and haze formation, conjunctival scarring, or any other ocular toxicity in any groups. h-MSC: human MSC; r-MSC: rabbit MSC.