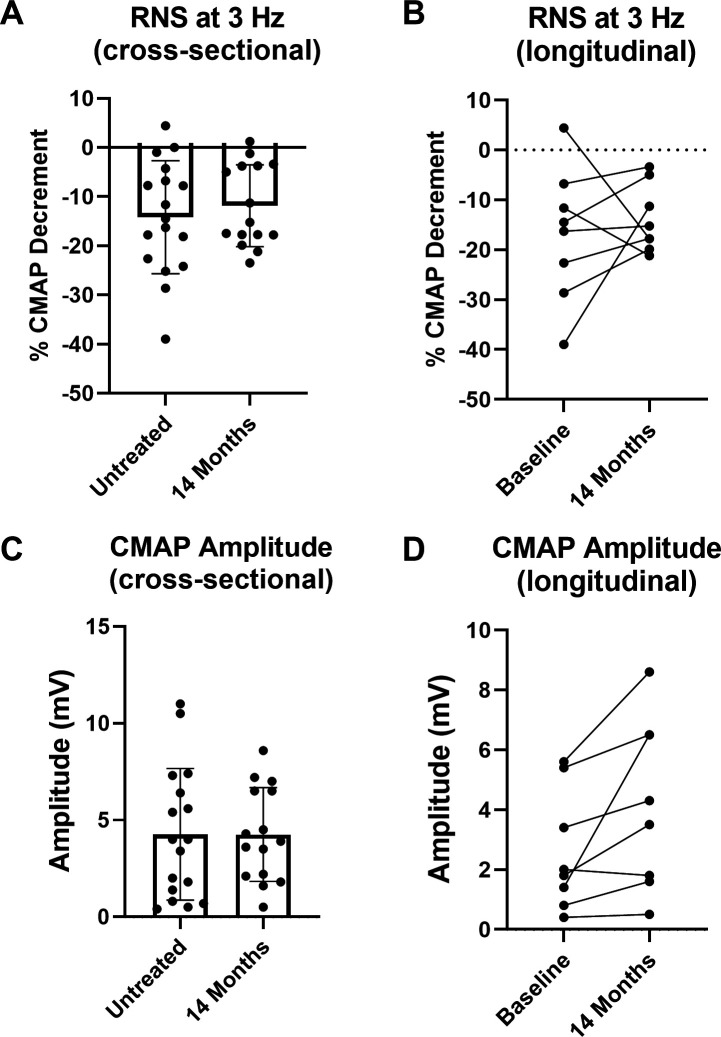
Figure 2.
Nusinersen does not result in overt improvement of CMAP decrement during RNS but does improve CMAP amplitude. (A) Cross-sectional analysis of CMAP decrement demonstrated no significant difference in assessments after 14 months of nusinersen (14 months of nusinersen treatment: −11.9%±8.3%, n=15, nine ambulatory) vs assessments prior to treatment (−14.2%±11.5% decrement, n=17, seven ambulatory) (unpaired t-test, p=0.5202). (B) Similarly, longitudinal assessment of a subset of participants (n=8, three ambulatory) demonstrated that CMAP amplitude decrement following 14 months of treatment (−13.9%±6.7%) was similar to baseline (−16.9%±13.4%) (paired t-test, p=0.5863). (C) Cross-sectional analysis of CMAP amplitude demonstrated no significant difference after 14 months of treatment (4.3±2.4 mV, n=15, nine ambulatory) vs assessments prior to treatment (4.3±3.4 mV, n=17, seven ambulatory) (unpaired t-test, p=0.9871). (D) In contrast, CMAP amplitude was significantly improved with nusinersen when analysed in a subset of participants (n=8, three ambulatory) that underwent assessment prior to treatment (2.6±2.0 mV) and after 14 months of nusinersen (4.2±2.8 mV) (paired t-test, p=0.0383). CMAP, compound muscle action potential; RNS, repetitive nerve stimulation.