Fig. 3. PROMPT cartridge assay evaluation.
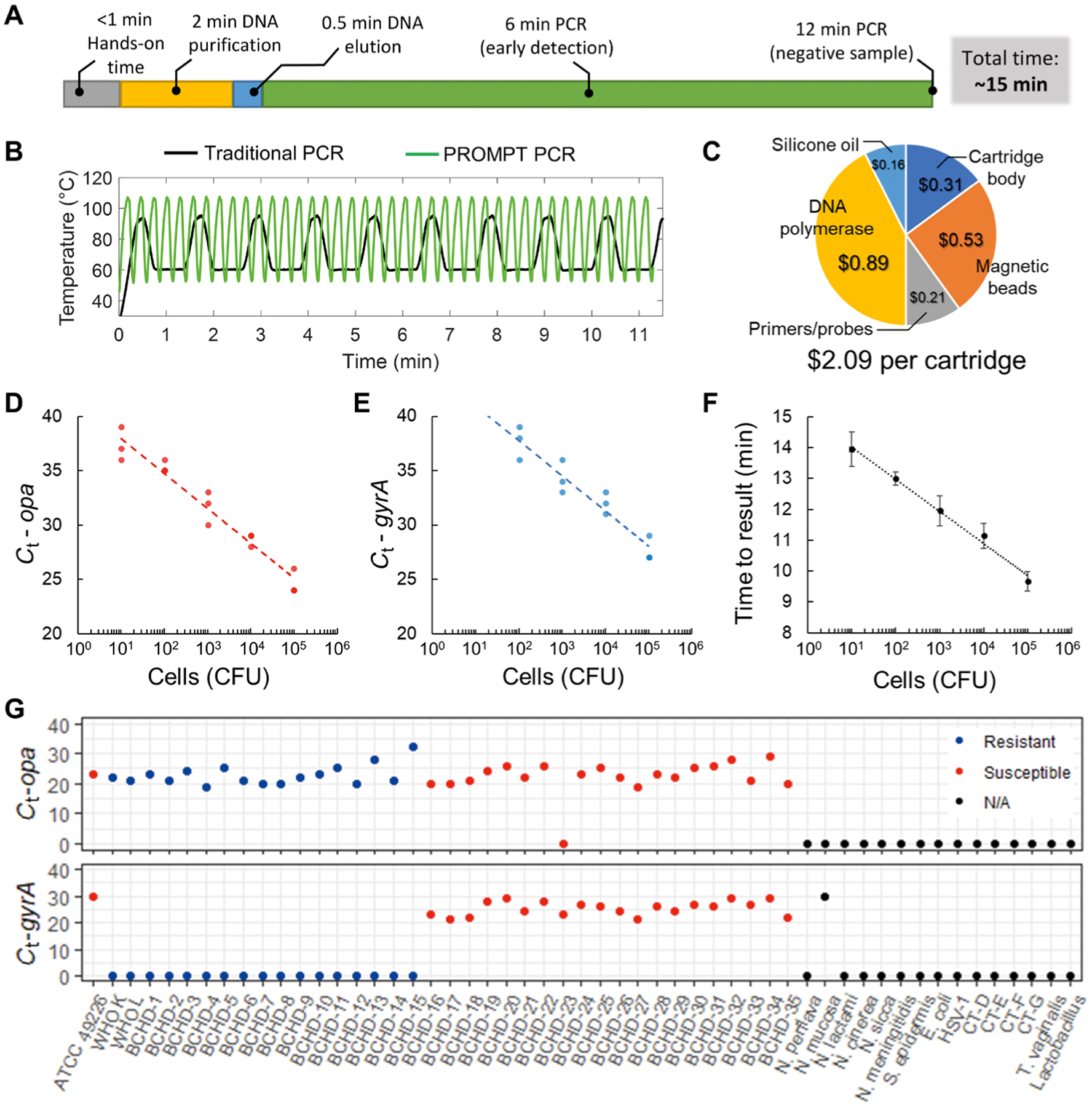
(A) Overall sample-to-answer workflow includes <1 min of hands-on time with DNA purification and with PCR amplification automated for a total time of 15 min. (B) Rapid thermocycling in the PROMPT instrument completes 40 cycles of PCR within 12 min during which a traditional thermocycling routine completes <10 cycles. (C) Cost breakdown for cartridge components. (D and E) Cartridge assay standard curves are shown using 10-fold serial dilutions run in triplicate (n = 3) from 1 to 105 CFU of N. gonorrhoeae input, with duplexed detection of opa (red) and wild-type gyrA (blue). (F) Time to result (minutes) using a live-detection algorithm versus N. gonorrhoeae CFU input to the cartridge. Error bars represent SD. (G) Cycle threshold (Ct) values for assay validation using reference strains of N. gonorrhoeae (ATCC, WHO), BCHD clinical isolates, related Neisseria species, and other organisms known to infect the urogenital tract. N. gonorrhoeae strains are color-coded by susceptibility to ciprofloxacin. No amplification is indicated by a Ct equal to 0.
